Challenges in the prevention of coagulase-negative ... Vishal.pdf · Challenges in the prevention...
Transcript of Challenges in the prevention of coagulase-negative ... Vishal.pdf · Challenges in the prevention...
Challenges in the prevention of coagulase-negative staphylococcal sepsis
in neonates
Ch
alleng
es in th
e preven
tion
of co
agu
lase-neg
ative staph
yloco
ccal sepsis in
neo
nates
Vish
al Hira
Vishal Hira
Uitnodiging
Voor het bijwonen van de openbare verdediging van het proefschrift
Challenges in the prevention of coagulase-negative staphylococcal sepsis in neonates
door
Vishal Hira
op woensdag 27 maart 2013om 13.30 uur
De plechti gheid zal plaatsvinden in de Prof. Andries Queridozaal (Eg-370) van het Erasmus MC, Dr. Molewaterplein 50 te Rott erdam
Na afl oop bent u van harte uitgenodigd voor de recepti e ter plaatse
Paranimfen:Archana HiraSebasti aan van Rijswijk
Vishal HiraAubeldomein 7c6229 EB [email protected]
Stellingen
behorende bij het proefschrift
Challenges in the prevention of coagulase-negative staphylococcal sepsis in neonates
1. Coagulase-negative staphylococci (CoNS) are an important cause of late-onset sepsis. (This thesis)
2. NICU personnel contributes to the spread of virulent CoNS. (This thesis)
3. Neonates who are colonised with resistant CoNS early after birth are of increased risk for developing CoNS late-onset sepsis. (This thesis)
4. SesC is a promising target for antibody mediated strategies against S. epidermidis. (This thesis)
5. A large proportion of late-onset sepsis can be prevented with proper hand hygiene. (This thesis)
6. Het concept van “humane eindpunten” in de proefdierkunde impliceert dat proefdieren menselijker worden behandeld dan mensen.
7. Education of women reduces child mortality. (Lancet 2010; 376: 959–74)
8. Artsen-microbioloog redden levens.
9. Randomized controlled trials hebben betrekking op groepen patiënten, niet op individuele patiënten.
10. De ideale dokter probeert zichzelf overbodig te maken.
11. Procrastination is the art of giving something the chance to solve itself.
Vishal Hira 27 maart 2013
Colofon
ISBN: 978-90-8891-583-3
Layout, cover design and photography: Vishal HiraPrinted by: Proefschriftmaken.nl || Uitgeverij BOXPress
Printing of this thesis was financially supported by ABN AMRO Bank N.V., BD Diagnostics, bioMérieux Benelux B.V., Erasmus University Rotterdam, Merck Sharp & Dohme B.V., the Netherlands Society of Medical Microbiology (NVMM) and the Royal Netherlands Society for Microbiology (KNVM)
© 2013 Vishal HiraAll rights reserved. No part of this thesis may be multiplied and/or published by means of print, photocopy, microfilm, or otherwise, without explicit permission of the author.Niets uit deze uitgave mag vermenigvuldigd en/of openbaar gemaakt worden door middel van druk, fotokopie of welke wijze dan ook, zonder voorafgaande schriftelijke toestemming van de auteur.
Challenges in the Prevention of Coagulase-Negative Staphylococcal
Sepsis in Neonates
Uitdagingen in de preventie van coagulase-negatieve stafylokokkensepsis in neonaten
Proefschrift
ter verkrijging van de graad van doctor aan de
Erasmus Universiteit Rotterdam op gezag van de
rector magnificus
Prof.dr. H.G. Schmidt
en volgens besluit van het College voor Promoties.
De openbare verdediging zal plaatsvinden op woensdag 27 maart 2013 om 13.30 uur
door
Vishal Hira
geboren te Paramaribo, Suriname
Promotiecommissie
Promotoren: Prof.dr. R. de Groot Prof.dr. P.W.M. Hermans
Overige leden: Prof.dr. A.J. van der Heijden Prof.dr. H.A. Verbrugh Prof.dr. J.D.F. Habbema Co-promotor: Dr. R.F. Kornelisse
Contents
Chapter 1
Chapter 2
Chapter 3
Chapter 4
Chapter 5
Chapter 6
Chapter 7
Appendix
General introduction
Clinical and molecular epidemiologic characteristics of coagulase-negative staphylococcal bloodstream infections in intensive care neonates
Coagulase-negative staphylococcal skin carriage among neonatal intensive care unit personnel: from population to infection
Colonization of neonates with coagulase-negative staphylococci4.1 Colonization dynamics of antibiotic-resistant coagulase- negative staphylococci in neonates4.2 Early colonization of neonates with antibiotic resistant coagulase-negative staphylococci is a risk factor for late-onset sepsis
Inhibition of Staphylococcus epidermidis biofilm formation5.1 Vaccination with SesC decreases Staphylococcus epidermidis biofilm formation5.2 Inhibition of Staphylococcus epidermidis biofilm formation by rabbit polyclonal antibodies against the SesC protein
Prevention of coagulase-negative staphylococcal late-onset sepsis in neonates
Summarizing discussion en Nederlandse samenvattingSummarizing discussion and future perspectivesNederlandse samenvatting en toekomstperspectieven
Contributing authorsDankwoordList of publicationsCurriculum vitae
8
16
30
4446
58
6668
88
108
122124128
132134136140141
Chapter 110
Gram-positive coagulase-negative staphylococci (CoNS) are a subgroup of staphylococci, which can be distinguished from Staphylococcus aureus and Staphylococcus intermedius by their lack of ability to produce coagulase. At present, the group consists of more than 40 known species (1). Half of them are isolated from humans. CoNS belong to the normal flora of the skin and mucosa. Several species are found at specific sites of the body. Staphylococcus capitis, for example, is mainly found on the head, while Staphylococcus auricularis is isolated almost exclusively from the external auditory meatus (2). Being commensal bacteria, CoNS were long thought to be non-pathogenic and were regarded as contaminants when found in specimens of human origin.
CoNS infections In 1958 Smith et. al. published a paper in which they demonstrated the potential pathogenic properties of CoNS in a collection of samples from patients with septicemia (Figure 1) (3). From then onwards, CoNS are thought to be a causative organism of bloodstream infections (4, 5), native and prosthetic valve endocarditis (6, 7), urinary tract infections (8, 9), eye infections (10, 11) and many other infections.
CoNS, and especially Staphylococcus epidermidis, are the most frequently isolated species in nosocomial bloodstream infections (NBI). Wisplinghoff et al. showed that CoNS were isolated in 31% of monomicrobial NBIs in the USA. The highest rates were found among the pediatric NBIs with rates up to 47% (5). Although the course of the infection is less fulminant than, for example, sepsis caused by S. aureus or Gram-negative organisms, CoNS sepsis causes significant morbidity, leading to a prolonged hospital stay and thus increased health care costs (12, 13). NBIs caused by CoNS are especially prevalent in patients with a weak immune response, like hemato-oncological patients and prematures.
CoNS sepsis in neonates CoNS are the major cause of late-onset sepsis (LOS) in neonatal intensive care units (NICUs). LOS is defined by the presence of clinical signs of sepsis after 72 hours of age (4, 14). Risk factors for CoNS associated LOS include patients with a low gestational age, a low birth weight and a history of prolonged intravascular catheterization (15, 16) (Figure 2).The increasing prevalence of CoNS infections is attributable to their increasing antibiotic resistance and their ability to form biofilms. The biofilm forming properties of CoNS enable them to grow on foreign bodies such as intravascular catheters. The most effective method for treating CoNS sepsis is to simply remove the catheter and thus remove the source of the bacteremia. However, intravascular catheters are necessary to administer nutrition
Figure 1. Publication of Smith et. al. in AMA Archives of Internal Medicine (1958)
General introduction 11
Ch
ap
ter
1
and medication and to perform blood gas analyses. In prematures, intravascular access is limited, especially in the smaller infants. Catheter removal is therefore not always possible and other modes of treatment of sepsis need to be applied. Administration of antibiotics to treat CoNS associated LOS has been successfully applied. However, as mentioned before, CoNS resistance to antibiotics has gradually increased over the years. In The Netherlands, where antibiotic policies are among the most restrictive of the world, methicillin resistance among CoNS doubled in the period 1989 to 1995 from 15 to 30% (17). In neonatal settings, it has been shown that up to 90% of the CoNS found in LOS are resistant to methicillin (18-20). Nowadays, vancomycin is often used as second choice antibiotic in CoNS LOS. The first case of vancomycin resistant Staphylococcus haemolyticus has already been described in 1987 (21). Ever since, there have been reports of vancomycin resistant CoNS, showing that vancomycin resistance in CoNS has become a serious problem (22-25). Even in The Netherlands, several vancomycin resistant CoNS have been found in both intensive care units, as well as in the community (26).
Biofilms Biofilm formation is one of the most important reasons for the success of CoNS NBIs in intensive care units. In a biofilm, bacteria are inherently resistant to host immune responses and antibiotics. There are several theories for the mechanisms behind this resistance. One is the inability for antibiotics to penetrate into the biofilm. A second theory is that cells inside a biofilm experience nutrition limitation and exist in a slow growing state and are therefore less susceptible to metabolically directed antibiotics (27).Since many patients have intravascular catheters, CoNS can grow in biofilms, and subsequently, spread into the bloodstream. A relation between the presence of intravascular catheters and CoNS LOS has been reported in several studies (14, 16). The process of biofilm formation and infection is illustrated in Figure 3. Biofilm formation consists of three phases. In the initial phase, CoNS attach intravascular catheters, using aspecific factors like van der Waal’s forces and hydrophobic interactions. In the second phase, the bacteria attach to extracellular matrix host proteins which have coated the catheter. Thereafter, the third phase follows, in which the attached bacteria accumulate and proliferate (28, 29). In the second and third phases, surface-exposed proteins play a major role. For example, the fibrinogen binding protein (Fbe) mediates attachment to fibrinogen in the second phase,
Figure 2. Infant with intravascular catheter at the NICU
Chapter 112
whereas the accumulation associated protein (Aap) is an important factor in accumulation in the third phase. It has been shown that antibodies against these proteins can inhibit biofilm formation (30, 31). The production of polysaccharide intercellular adhesion (PIA) is also thought to play an important role in the third phase. PIA is regulated by the ica operon and presence of the ica genes have been shown to be predictors for biofilm formation (32).
Prevention In the past, several efforts have been made to prevent CoNS associated LOS on NICUs. As transplacental transfer of antibodies start at approximately 28 weeks of gestation, several groups have studied the effect of prophylactic administration of intravenous immune globulins (IVIG) to prematures with CoNS associated LOS. In these studies, only a slight reduction in the incidence of CoNS associated LOS was observed (33, 34). Antibodies against CoNS lipoteichoic acid, PIA and Fbe have opsonophagocytotic properties in vitro (35-38). In 2009, a phase 1/2 double-blind, placebo-controlled study of a monoclonal antilipoteichoic acid antibody showed no clinical effect of prophylactic administration of the antibody to very-low-birth-weigth neonates (39).Prevention of biofilm formation may also be an effective measurement for the prevention of CoNS associated LOS. As mentioned before, in vitro studies have shown that antibodies against Fbe and AAP inhibit biofilm formation. Other strategies include blocking biofilm formation with catheter lock solutions, with a fish protein coating and by impregnation of catheters with phages and incorporation of phages in hydrogel coating of catheter (40-42). Although some of these strategies are promising, clinical applications are not yet available.Another important factor in prevention of CoNS associated LOS is the prevention of cross-contamination of CoNS strains. Studies have shown that CoNS outbreaks on NICU are usually caused by clonal strains (16, 19, 43-45). CoNS are thought to be transmitted to the neonates by NICU personnel, since they have the most intensive contact with prematures. Improving
Figure 3. Biofilm formation on an intravascular catheter. A) CoNS on the skin attach to the catheter by aspecific factors like hydrophobic interactions. B) Inside the vessel, the catheter is coated by host matrix proteins like fibrin and fibrinogen. Using surface proteins like Fbe (SdrG) and AtlE, CoNS attach to these host proteins. C) Polysaccharide, specific proteins like AAP and accessory macromolecules provide intercellular aggregation and bacteria proliferate and accumulate in multilayered cell clusters. D) The biofilm matures and detaches. Mechanism are poorly understood, but quorum sensing controlled expression of detergent-like peptides and proteolytic activity is might be involved. (Reprinted by permission from Macmillan Publishers Ltd: Nature Reviews Microbiology (Nat Rev Microbiol. 2009 Aug;7(8):555-67), copyright 2009) (28)
General introduction 13
Ch
ap
ter
1
hand hygiene on NICUs is therefore considered an important strategy in the prevention of CoNS associated LOS. Although the importance of hand washing is generally acknowledged, compliance to hand washing protocols still seems to be difficult (46).
Aims and outline of this thesis As antibiotic resistance in CoNS is rapidly increasing, prevention is the most important factor in the battle against CoNS associated LOS on NICUs. For the prevention of cross-contamination and biofilm formation, it is important to have a detailed understanding of the underlying mechanisms in order to design adequate strategies. In this thesis we therefore aim to study the transfer of infecting CoNS strains from personnel to patients, to assess neonatal colonization with infecting CoNS and to unravel important factors in biofilm formation. In chapter 2, we study the incidence of CoNS associated LOS at a large NICU, as well as the characteristics of CoNS sepsis patients. We also assess the molecular epidemiology, biofilm forming properties and antibiotic resistance of isolated CoNS strains. Furthermore we study the use of restricted fragment end labeling for the molecular typing of CoNS.In Chapter 3 we report the results of a study comparing CoNS isolated from NICU personnel with community CoNS isolates and sepsis isolates, in the extent of antibiotic resistance and biofilm forming properties. Furthermore, effects of absence of personnel from the NICU on isolates from personnel are studied as well.Development of antibiotic resistance in CoNS isolated from the skin and gut of neonates is studied in chapter 4, whereas chapter 5 focuses on a possible relation between early CoNS colonization and late-onset sepsis. Chapter 6 describes the identification of surface-exposed proteins of S. epidermidis and studies involvement of several known and unknown surface/exposed proteins in biofilm formation. One of these proteins, SesC, is extensively studied in chapter 6 and 7 for its use as a target in prevention and treatment of S. epidermidis biofilms. Chapter 8 reviews different strategies for prevention of CoNS sepsis in neonates, discussing usefulness of existing strategies and possible strategies based on published literature and this thesis.
Chapter 114
References
1. Widerstrom M, Wistrom J, Sjostedt A, Monsen T. Coagulase-negative staphylococci: update on the molecular epidemiology and clinical presentation, with a focus on Staphylococcus epidermidis and Staphylococcus saprophyticus. Eur J Clin Microbiol Infect Dis. 2012;31:7-20.
2. Huebner J, Goldmann DA. Coagulase-negative staphylococci: role as pathogens. Annual review of medicine. 1999;50:223-236.
3. Smith IM, Beals PD, Kingsbury KR, Hasenclever HF. Observations on Staphylococcus albus septicemia in mice and men. AMA. 1958;102:375-388.
4. Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285-291.
5. Wisplinghoff H, Bischoff T, Tallent SM, et al. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309-317.
6. Chu VH, Cabell CH, Abrutyn E, et al. Native valve endocarditis due to coagulase-negative staphylococci: report of 99 episodes from the International Collaboration on Endocarditis Merged Database. Clin Infect Dis. 2004;39:1527-1530.
7. Wang A, Athan E, Pappas PA, et al. Contemporary clinical profile and outcome of prosthetic valve endocarditis. Jama. 2007;297:1354-1361.
8. Grude N, Tveten Y, Kristiansen BE. Urinary tract infections in Norway: bacterial etiology and susceptibility. A retrospective study of clinical isolates. Clin Microbiol Infect. 2001;7:543-547.
9. Mathai D, Jones RN, Pfaller MA. Epidemiology and frequency of resistance among pathogens causing urinary tract infections in 1,510 hospitalized patients: a report from the SENTRY Antimicrobial Surveillance Program (North America). Diagnostic microbiology and infectious disease. 2001;40:129-136.
10. Benz MS, Scott IU, Flynn HW, Jr., Unonius N, Miller D. Endophthalmitis isolates and antibiotic sensitivities: a 6-year review of culture-proven cases. American journal of ophthalmology. 2004;137:38-42.
11. Tarabishy AB, Hall GS, Procop GW, Jeng BH. Bacterial culture isolates from hospitalized pediatric patients with conjunctivitis. American journal of ophthalmology. 2006;142:678-680.
12. Gray JE, Richardson DK, McCormick MC, Goldmann DA. Coagulase-negative staphylococcal bacteremia among very low birth weight infants: relation to admission illness severity, resource use, and outcome. Pediatrics. 1995;95:225-230.
13. Payne NR, Carpenter JH, Badger GJ, Horbar JD, Rogowski J. Marginal increase in cost and excess length of stay associated with nosocomial bloodstream infections in surviving very low birth weight infants. Pediatrics. 2004;114:348-355.
14. Klingenberg C, Aarag E, Ronnestad A, et al. Coagulase-negative staphylococcal sepsis in neonates. Association between antibiotic resistance, biofilm formation and the host inflammatory response. Pediatr Infect Dis J. 2005;24:817-822.
15. Healy CM, Palazzi DL, Edwards MS, Campbell JR, Baker CJ. Features of invasive staphylococcal disease in neonates. Pediatrics. 2004;114:953-961.
16. Vermont CL, Hartwig NG, Fleer A, et al. Persistence of clones of coagulase-negative staphylococci among premature neonates in neonatal intensive care units: two-center study of bacterial genotyping and patient risk factors. Journal of clinical microbiology. 1998;36:2485-2490.
17. de Neeling AJ, van Leeuwen WJ, Schouls LM, et al. Resistance of staphylococci in The Netherlands: surveillance by an electronic network during 1989-1995. J Antimicrob Chemother. 1998;41:93-101.
18. De Giusti M, Pacifico L, Tufi D, et al. Phenotypic detection of nosocomial mecA-positive coagulase-negative staphylococci from neonates. J Antimicrob Chemother. 1999;44:351-358.
19. Krediet TG, Mascini EM, van Rooij E, et al. Molecular epidemiology of coagulase-negative staphylococci causing sepsis in a neonatal intensive care unit over an 11-year period. Journal of clinical microbiology. 2004;42:992-995.
20. Raimundo O, Heussler H, Bruhn JB, et al. Molecular epidemiology of coagulase-negative staphylococcal bacteraemia in a newborn intensive care unit. The Journal of hospital infection. 2002;51:33-42.
21. Schwalbe RS, Stapleton JT, Gilligan PH. Emergence of vancomycin resistance in coagulase-negative staphylococci. The New England journal of medicine. 1987;316:927-931.
22. Center KJ, Reboli AC, Hubler R, Rodgers GL, Long SS. Decreased vancomycin susceptibility of coagulase-negative staphylococci in a neonatal intensive care unit: evidence of spread of Staphylococcus warneri. Journal of clinical microbiology. 2003;41:4660-4665.
23. Krcmery V, Jr., Trupl J, Drgona L, et al. Nosocomial bacteremia due to vancomycin-resistant Staphylococcus epidermidis in four patients with cancer, neutropenia, and previous treatment with vancomycin. Eur J Clin Microbiol Infect Dis. 1996;15:259-261.
General introduction 15
Ch
ap
ter
1
24. Palazzo IC, Araujo ML, Darini AL. First report of vancomycin-resistant staphylococci isolated from healthy carriers in Brazil. Journal of clinical microbiology. 2005;43:179-185.
25. Sanyal D, Johnson AP, George RC, Cookson BD, Williams AJ. Peritonitis due to vancomycin-resistant Staphylococcus epidermidis. Lancet. 1991;337:54.
26. SWAB. NethMap 2012. National Institute for Public Health and the Environment; 2012.27. Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science.
1999;284:1318-1322.28. Otto M. Staphylococcus epidermidis--the ‘accidental’ pathogen. Nat Rev Microbiol. 2009;7:555-567.29. von Eiff C, Peters G, Heilmann C. Pathogenesis of infections due to coagulase-negative staphylococci. Lancet
Infect Dis. 2002;2:677-685.30. Pei L, Flock JI. Functional study of antibodies against a fibrogenin-binding protein in Staphylococcus
epidermidis adherence to polyethylene catheters. The Journal of infectious diseases. 2001;184:52-55.31. Sun D, Accavitti MA, Bryers JD. Inhibition of biofilm formation by monoclonal antibodies against
Staphylococcus epidermidis RP62A accumulation-associated protein. Clinical and diagnostic laboratory immunology. 2005;12:93-100.
32. Arciola CR, Baldassarri L, Montanaro L. Presence of icaA and icaD genes and slime production in a collection of staphylococcal strains from catheter-associated infections. Journal of clinical microbiology. 2001;39:2151-2156.
33. Baker CJ, Melish ME, Hall RT, et al. Intravenous immune globulin for the prevention of nosocomial infection in low-birth-weight neonates. The Multicenter Group for the Study of Immune Globulin in Neonates. The New England journal of medicine. 1992;327:213-219.
34. Weisman LE, Stoll BJ, Kueser TJ, et al. Intravenous immune globulin prophylaxis of late-onset sepsis in premature neonates. The Journal of pediatrics. 1994;125:922-930.
35. Maira-Litran T, Kropec A, Abeygunawardana C, et al. Immunochemical properties of the staphylococcal poly-N-acetylglucosamine surface polysaccharide. Infect Immun. 2002;70:4433-4440.
36. Rennermalm A, Nilsson M, Flock JI. The fibrinogen binding protein of Staphylococcus epidermidis is a target for opsonic antibodies. Infect Immun. 2004;72:3081-3083.
37. Vernachio JH, Bayer AS, Ames B, et al. Human immunoglobulin G recognizing fibrinogen-binding surface proteins is protective against both Staphylococcus aureus and Staphylococcus epidermidis infections in vivo. Antimicrob Agents Chemother. 2006;50:511-518.
38. Weisman LE. Coagulase-negative staphylococcal disease: emerging therapies for the neonatal and pediatric patient. Current opinion in infectious diseases. 2004;17:237-241.
39. Weisman LE, Thackray HM, Garcia-Prats JA, et al. Phase 1/2 double-blind, placebo-controlled, dose escalation, safety, and pharmacokinetic study of pagibaximab (BSYX-A110), an antistaphylococcal monoclonal antibody for the prevention of staphylococcal bloodstream infections, in very-low-birth-weight neonates. Antimicrob Agents Chemother. 2009;53:2879-2886.
40. Lu TK, Collins JJ. Dispersing biofilms with engineered enzymatic bacteriophage. Proc Natl Acad Sci U S A. 2007;104:11197-11202.
41. Shanks RM, Sargent JL, Martinez RM, Graber ML, O’Toole GA. Catheter lock solutions influence staphylococcal biofilm formation on abiotic surfaces. Nephrol Dial Transplant. 2006;21:2247-2255.
42. Vejborg RM, Klemm P. Blocking of bacterial biofilm formation by a fish protein coating. Appl Environ Microbiol. 2008;74:3551-3558.
43. Foka A, Chini V, Petinaki E, et al. Clonality of slime-producing methicillin-resistant coagulase-negative staphylococci disseminated in the neonatal intensive care unit of a university hospital. Clin Microbiol Infect. 2006;12:1230-1233.
44. Tan TQ, Musser JM, Shulman RJ, et al. Molecular epidemiology of coagulase-negative Staphylococcus blood isolates from neonates with persistent bacteremia and children with central venous catheter infections. The Journal of infectious diseases. 1994;169:1393-1397.
45. Villari P, Sarnataro C, Iacuzio L. Molecular epidemiology of Staphylococcus epidermidis in a neonatal intensive care unit over a three-year period. Journal of clinical microbiology. 2000;38:1740-1746.
46. Harris AD, Samore MH, Nafziger R, et al. A survey on handwashing practices and opinions of healthcare workers. The Journal of hospital infection. 2000;45:318-321.
Chapter
2
Clinical and molecularepidemiological
characteristics ofcoagulase-negative
staphylococcalbloodstream infections in
intensive care neonates
Vishal HiraMarcel Sluijter
Silvia EstevãoDeborah Horst-Kreft
Alewijn OttRonald de Groot
Peter W. M. HermansRené F. Kornelisse
Pediatr Infect Dis J. 2007;26(7):607-12
16
Chapter
2
Clinical and molecularepidemiological
characteristics ofcoagulase-negative
staphylococcalbloodstream infections in
intensive care neonates
Vishal HiraMarcel Sluijter
Silvia EstevãoDeborah Horst-Kreft
Alewijn OttRonald de Groot
Peter W. M. HermansRené F. Kornelisse
Pediatr Infect Dis J. 2007;26(7):607-12
Chapter 218
Abstract
Objectives This study aimed to determine clinical characteristics of coagulase-negative staphylococcal (CoNS) sepsis in neonates, to assess the molecular epidemiology and biofilm forming properties of isolated strains, and to assess antibiotic susceptibility of clonal compared with incidentally occurring strains.
Methods We performed a retrospective study on late-onset CoNS sepsis in infants in the neonatal intensive care unit of a Dutch university hospital in 2003. CoNS isolates were genotyped by restriction fragment end labeling and pulsed-field gel electrophoresis. Resistance profiles, biofilm production, and the presence of mecA and icaA were determined.
Results Twenty-six percent of all 339 infants developed late-onset sepsis, 66% of these with CoNS sepsis. Eighty-six percent of all CoNS sepsis occurred in very low birth weight infants. Sixty-six CoNS strains were isolated. In multivariate analysis, small for gestational age and prolonged hospitalization were associated with CoNS sepsis. Among 3 restriction fragment end labeling clusters, we found 1 large cluster comprising 32% of the isolates. Biofilm producing Staphylococcus epidermidis were more frequently icaA positive than nonbiofilm formers (74% vs. 12%; P < 0.001). In other species, this association was not found. Nearly all isolates were resistant to antibiotics. MecA was present in 87% of the isolates. Multiresistance occurred in 77% of all strains and in 73% of clustered strains. There was significantly less multiresistance among the largest cluster.
Conclusions Small for gestational age and prolonged hospitalization were associated with CoNS sepsis. The icaA gene is a predictor for biofilm formation in S. epidermidis, but not in other species. Multiresistance is not associated with clonality.
CoNS sepsis in neonates 19
Ch
ap
ter
2
Introduction
Late-onset sepsis (LOS) is common in infants admitted to a neonatal intensive care unit (NICU). The current greater viability of premature infants has even led to a higher incidence of these infections. Previous studies have reported incidences ranging from 5% to 32% (1). LOS is associated with higher morbidity, mortality, and cost of resources, mainly because of the prolonged hospitalization (2-4). Important risk factors are immaturity of the immune system and invasive procedures (5-7). An additional risk factor is poor infection control (8). The major causative pathogens are common skin commensals such as coagulase-negative staphylococci (CoNS), which are isolated in approximately 50% of LOS (1,5). These infections are associated with low birth weight (BW), low gestational age (GA), a history of prolonged intravascular catheterization, and longer hospital stay. (9) Molecular epidemiology studies, mainly using pulsed-field gel electrophoresis (PFGE), have shown that CoNS isolated from a NICU often belong to dominant clones (7,10-12). Over the past decades, the isolated strains have increasingly become multiresistant to antibiotics (13). The biofilm forming capacities of CoNS are thought to be the main virulence factor. Adhering to polymer surfaces, for instance of vascular catheters, CoNS can grow rapidly and spread into the bloodstream. The biofilm forming process is mostly governed by polysaccharide intercellular adhesin synthesis, which in turn is mediated by the icaADBC operon. The presence of this operon is therefore thought to be associated with virulent CoNS strains (14).We aimed: 1. to determine the incidence of CoNS sepsis at a large NICU in 2003; 2. to determine clinical characteristics of those patients who acquired a CoNS sepsis; 3. to assess the molecular epidemiology of isolated CoNS strains; 4. to assess biofilm forming properties of the isolated CoNS, and 5. to assess whether clonal strains are less susceptible to antibiotics than are sporadic strains.
Materials & Methods
Description of Patients A retrospective study was conducted at the NICU of the Erasmus MC–Sophia Children’s Hospital in Rotterdam, the Netherlands. This NICU consists of 3 wards with 9 level III beds each. About 550 infants are admitted yearly, of which one-third have very low birth weight (VLBW) (<1500 g). Eligible for inclusion were those infants admitted for more than 72 hours in the year 2003. Demographical and clinical data were obtained from computerized databases and medical records. These included gender, BW, GA, procedures and complications during hospitalization, number of CoNS sepsis episodes, age at infection, and isolated organisms.LOS was defined as described by Stoll et. al. (6) and Klingenberg et. al. (15) and required clinical signs of sepsis after 72 hours of age, one or more positive blood cultures and a raised CRP (>10 mg/L) within 2 days of blood culture. Whenever CoNS and another pathogen were identified in the same blood culture, only the other pathogen was recorded.
Blood Cultures Blood cultures were performed with the BacTec 9240 System using pediatric bottles (Becton Dickinson, Meylan, France). Per bottle, at least 0.5 mL blood was inoculated. Bottles were incubated at 37°C for a maximum of 7 days. Culture purity was assessed by Gram stain and
Chapter 220
visual examination of colony forming units at various agar plates. CoNS isolates were stored in glycerol-containing liquid media at -80°C in the hospital microbiology laboratory.
Antibiotic Resistance Susceptibility of bacterial isolates to the following antibiotics was assessed using automated VITEK system (bioMe´rieux, Marcy l’Etoile, France): penicillin, flucloxacillin, cefuroxime, tobramycin, cotrimoxazol, ciprofloxacin, ofloxacin, clindamycin, erythromycin, rifampin, tetracycline and vancomycin. In addition, we determined the presence of the mecA gene encoding for methicillin resistance by mecA polymerase chain reaction (PCR) (16). Multiresistance was defined as resistance to 2 or more antibiotic groups.
Bacterial Culture All staphylococcal isolates were cultured from -80°C glycerol stock on Columbia blood agar or Tryptic soy agar and grown at 37°C overnight.
Molecular Typing Restriction fragment end labeling (RFEL) was performed by the method of van Steenbergen et al (17) as adapted by Hermans et al. (18). In short, cetyl trimethyl ammonium bromide purified staphylococcal DNA was digested by restriction enzyme EcoRI. DNA restriction fragments were end labeled at 72°C with [α-32P]dATP using thermostable DNA polymerase (Integro, Leuvenheim, The Netherlands) and electrophoretically separated on a polyacrylamide gel. The gel was vacuum dried (HBI, Saddlebrook, N.Y.), and exposed overnight to Fuji RX. Films were scanned and analyzed by use of BioNumerics 3 (Applied Maths, Sint-Martens-Latem, Belgium) software using the Unweighted Pair Group Method with Arithmetic mean algorithm with the Jaccard coefficient and a band position tolerance of 1.2%. Band positions were assigned manually. Normalization was performed on the Staphylococcus epidermidis ATCC 12228 strain, which was run between every 5 samples. Relations between CoNS strains were visualized in a dendrogram as described before (19). PFGE was performed as described by Vermont et. al. (7) Gels were stained with ethidium bromide, photographed and analyzed by use of BioNumerics 3 software using the Unweighted Pair Group Method with Arithmetic mean algorithm with the Dice coefficient and a band position tolerance of 1.2%. Normalization was performed on the 50 kb Lambda Ladder PFG marker (New England Biolabs, Ipswich, MA, USA), which was run between every 5–6 strains. Strains with at least 70% genetic similarity were assigned to 1 cluster. Using PFGE, RFEL typing was validated by analysis of the concordance between these 2 techniques in BioNumerics 3 software. The concordance was visualized in a correlation curve from which we determined our definition of relatedness in RFEL.
Species Identification On all isolates we performed species identification using automated VITEK 2 system (bioMerieux). Internal transcriber spacer (ITS)-PCR was performed as described by Shittu et. al (20).
Biofilm Production Semiquantitative determination of biofilm production was adapted from the method of Christensen et. al. (21). In short, 200 μL of each strain in brain heart infusion broth was inoculated overnight in 4 parallel wells in a polystyrene microtiter plate. After staining with
CoNS sepsis in neonates 21
Ch
ap
ter
2
crystal violet and drying, the biofilm was solved in 160 μL 33% glacial acetic acid (22) and the optical density (OD) was measured spectrophotometrically at 595 nm. The assay was independently repeated twice, the highest and lowest OD595 values were excluded from analysis and the remaining 10 values were averaged. We used S. epidermidis ATCC strain 12228 and S. epidermidis strain 4104 as negative and positive controls, respectively. Strains with OD595 <0.20, 0.20 ≤OD595 ≤1.0, and OD595 >1.0 were defined as biofilm negative, weak and strong biofilm formers, respectively (23). PCR on icaA, as a marker for the ica operon, was performed as previously reported (23).
Statistical AnalysisStatistics were performed using SPSS for Windows software, version 11 (Chicago, IL). χ2 test was used for univariate significance testing of categorical data. Differences between other variables were analyzed by the nonparametric (2-tailed) Mann–Whitney U test. Multivariate analysis was done with binary logistic regression. Variables that were associated with CoNS sepsis at a P value of <0.25 were included into the multivariate analysis. For determining the concordance between RFEL and PFGE, a Kendall’s tau correlation curve was determined using a third degree regression. P values of <0.05 were considered significant.
Results
Characteristics of Patients A total of 548 patients were admitted during the study period. However, 35 were immediately transferred to another NICU center, because all facilities were occupied. Of the remaining 513 children, 14 died and 160 were discharged within 72 hours after birth. Consequently, analysis was for 339 children. Of these, 152 (45%) were VLBW infants. Eighty-six of all 339 children (26%) developed 107 episodes of LOS. Gram-positive agents were the most frequent organisms (92% of all episodes). CoNS accounted for 66 (62%) episodes in 57 (66%) of the 86 children. Five children suffered twice from CoNS infection, 2 thrice. These 57 children all had an intravascular catheter in place. These 66 CoNS isolates were obtained by blood culture from a peripheral vein in 54 episodes, from an arterial catheter in 4 episodes and from both in 7 episodes. The source of the blood culture was unknown in 1 episode. Eighty-six percent of the children with a CoNS sepsis were VLBW infants. S. aureus, the second most common Gram-positive agent, was isolated in 25 (23%) episodes. Gram-negative agents accounted for 6 (6%) episodes. Three infants had a sepsis episode because of multiple organisms. The 152 included VLBW infants had a significantly lower median GA (28 weeks vs. 30 weeks, P < 0.001) and median BW (1000 g vs. 1275 g, P < 0.001) than those discharged or died within 72 hours after birth. Seventy (46%) infants developed 1 or more culture positive bloodstream infections, 49 (70%) of these with CoNS. In addition, we have observed that 87% of the CoNS sepsis occurred in the first 14 days of admission. Table 1 shows characteristics of VLBW children broken down into 2 categories: no sepsis at all, and CoNS sepsis. Logistic regression analysis, adjusting for patent ductus arteriosus, conventional ventilation and continuous positive airway pressure revealed that small for gestational age (SGA) children and a hospitalization of more that 14 days were associated with CoNS sepsis with an odds ratio of 2.7 (P = 0.03) and 6.0 (P = 0.006), respectively.
Chapter 222
Molecular Epidemiology of the CoNS Isolates Sixty-six CoNS sepsis strains met the sepsis criteria. Four were not available and 2 were not typable. The remaining 60 were genotyped by both RFEL and PFGE. To assess similarity between these 2 techniques a correlation plot was made (Figure 1). The plot displayed an r value of 74% (P = 0.01). Because 70% PFGE similarity corresponded to 88% RFEL similarity, we assigned strains with at least 88% RFEL similarity to 1 cluster. RFEL patterns of the 60 strains are shown in Figure 2. Genetic diversity ranged from 0% to 65%. Three large clusters, comprising 55% of all strains, could be identified. The number of strains in cluster 1 was 19 (32%).Three major clusters were spread over the year; yet 4 of the 6 strains isolated in May and 4 of the 7 strains isolated in December belonged to respectively cluster 2 and 3, suggesting horizontal transmission (Figure 2). None of the clusters were related to a specific unit.
Species Identification VITEK 2 typing showed 5 different species: S. epidermidis, S. haemolyticus, S. capitis, S. hominis and S. warneri (Figure 2). Of these species, S. epidermidis was most frequently isolated (58%), followed by S. haemolyticus (18%), S. capitis (15%), S. hominis (8%) and S. warneri (2%). Using ITS-PCR, 5 patterns could be identified. In 5 cases, these 2 techniques did not match. RFEL typing showed good clustering of the different species, although in 3 cases there was discrepancy between VITEK 2 identification and RFEL. The 3 largest clusters comprised large parts of the 3 most frequent species. Cluster 1 comprised 53% of the S. epidermidis, cluster 2 64% of the S. haemolyticus and cluster 3 56% of the S. capitis.
Table 1. Characteristics of VLBW infants without sepsis, and with CoNS sepsis.
Without sepsis With CoNS sepsis P-value (n=82) (n=49) Univariate
Male (%) 46.3 49.0 0.770
Birth weight (g) 1040 1000 0.252
Gestational age (weeks) 28 28 0.833
SGA (%) 18.3 34.7 0.047
PDA (%) 40.2 57.1 0.061
NEC (%) 6.1 10.2 0.392
Age at first infection (days) - 8 -
Central venous catheters (%) 58.5 65.3 0.442
Conventional ventilation > 7 days (%) 26.8 42.9 0.059
CPAP > 7 days (%) 40.2 65.3 0.006
Hospitalization > 14 days (%) 45.1 79.6 <0.001
Death (%) 17.1 10.2 0.280
Data are expressed as median, or percentages. Abbreviations: SGA: small for gestational age; PDA: patent ductus arteriosus; NEC: necrotizing enterocolitis (Bell stage IIA or greater); CPAP: continuous positive airway pressure.
CoNS sepsis in neonates 23
Ch
ap
ter
2
Figure 1. Analysis of concordance between RFEL and PFGE. Each dot represents the similarity between 2 isolates. The third degree regression line is depicted, as well as the 95% borders. Using Kendall’s tau correlation, an r-value of 74% (P 0.01) was calculated. 70% PFGE similarity correlated with 88% RFEL similarity.
Biofilm Formation As presented in Figure 2, 30 strains (48%) formed a biofilm: 12 of these were strong biofilm formers, the other 18 were weak producers. Eighteen of these biofilm producing strains (64%) belonged to 1 of the 3 clusters. S. epidermidis was frequently associated with the strong biofilm forming phenotype compared with the non-S. epidermidis strains (31% vs. 4%; P = 0.009), whereas S. haemolyticus was more frequently associated with the weak biofilm forming phenotype compared with the non-S. haemolyticus strains (73% vs. 20%; P < 0.001). The icaA PCR was positive in 26 strains (42%), with 17 strains (65%) belonging to 1 of the clusters. Compared with other species, S. haemolyticus was significantly less icaA PCR positive (9% vs. 49%; P = 0.015), whereas S. capitis was more frequently icaA PCR positive (89% vs. 34%; P = 0.002). Strong biofilm producers were more often icaA PCR positive than other strains (92% vs. 30%; P < 0.001). Among S. epidermidis, biofilm producers were more often icaA PCR positive than nonbiofilm producers (74% vs. 12%; P < 0.001). Among S. capitis, however, biofilm producers were less often icaA PCR positive than nonbiofilm producers (50% vs. 100%; P = 0.047). GA, BW, and age at infection were not associated with the biofilm forming or icaA positive strains
Antibiotic Resistance Isolates were especially resistant to β-lactam antibiotics. All but 2 strains were resistant to penicillin. One strain was susceptible to all antibiotics. At 90% overall rate, flucloxacillin resistance equalled cefuroxim resistance. This rate was in accordance with the presence of the mecA gene, which was found in 87% of the strains. All strains were sensitive to vancomycin. Multiresistance was found in 77% of the strains (Figure 2). Seventy-three percent of the clustered strains were multiresistant, as well as 82% of the nonclustered
Chapter 224
strains. Strains from cluster 1 were significantly less often multiresistant than other strains (58% vs. 86%; P = 0.015). Compared with other species, S. epidermidis was significantly less often multiresistant (67% vs. 92%; P = 0.017), whereas S. haemolyticus was significantly more often multiresistant (100% vs. 73%; P = 0.048). A significant difference in multiresistant strains between S. epidermidis and non-S. haemolyticus strains was not found (67% vs. 87%; P = 0.145). Among S. epidermidis, presence of the icaA gene was positively associated with a positive MecA PCR (100% vs. 75%; P = 0.031).
Figure 2. RFEL patterns and other characteristics of 62 CoNS isolates. The dotted line in the dendrogram depicts the 88% similarity border. *) : not typable.
Cluster Number of Ward Month of ITS VITEK IcaA Biofilm MecA MultiresistantStrains Isolation PCR Detemination PCR Formation PCR Phenotype
2 October B S. epidermidis - Negative + Yes3 November B S. haemolyticus - Weak + Yes3 April B S. haemolyticus - Weak + Yes3 May B S. haemolyticus + Weak + Yes2 May B S. haemolyticus - Weak + Yes1 May B S. haemolyticus - Weak + Yes1 December B S. haemolyticus - Weak + Yes1 May B S. haemolyticus - Strong + Yes1 September B S. haemolyticus - Weak + Yes2 August B S. haemolyticus - Negative + Yes1 September D S. hominis - Negative - No1 March A S. epidermidis + Strong + Yes3 March A S. epidermidis + Negative + Yes3 January A S. epidermidis + Strong + Yes2 November A S. epidermidis + Strong + Yes3 January A S. epidermidis + Strong + No1 March A S. epidermidis - Negative + No1 April A S. epidermidis - Negative + Yes2 July A S. epidermidis + Strong + No2 February A S. epidermidis - Weak + Yes
1 19 2 February A S. epidermidis - Weak + Yes3 July A S. epidermidis - Negative + Yes2 February A S. epidermidis - Negative + No3 December C S. epidermidis + Strong + No2 March A S. epidermidis + Strong + Yes2 August A S. epidermidis - Negative - No1 September A S. epidermidis - Weak - No3 July A S. epidermidis + Strong + Yes1 July A S. epidermidis + Strong + No1 December A S. epidermidis - Negative + Yes3 January A S. epidermidis + Weak + Yes1 August A S. epidermidis - Weak - No2 October A S. epidermidis - Negative + No1 June A S. epidermidis + Strong + Yes1 February A S. epidermidis - Weak - Yes1 November A S. epidermidis - Negative - Yes2 September A S. epidermidis + Negative + Yes1 September A S. epidermidis + Weak + Yes2 March A S. epidermidis + Weak + Yes1 February A S. epidermidis - Negative + Yes2 August A S. epidermidis - Negative + No2 August A S. epidermidis - Negative + No3 October A S. epidermidis - Negative + Yes1 October A S. hominis - Negative + Yes3 May A S. epidermidis - Negative + Yes1 March A S. epidermidis + Strong + Yes3 November A S. epidermidis - Negative + Yes1 March C S. capitis + Negative + Yes2 July C S. capitis + Negative + Yes2 December C S. warneri + Negative - Yes3 December C S. capitis + Negative + Yes1 December C S. capitis + Negative + Yes2 February C S. capitis + Negative + Yes1 May C S. capitis + Negative + Yes1 December C S. capitis + Negative + No1 November C S. capitis - Weak + Yes2 February D S. capitis + Weak - Yes3 October E S. hominis - Negative + Yes1 October E S. hominis - Negative + Yes1 July E S. hominis - Negative + Yes
*) 1 August B S. haemolyticus + Strong + Yes*) 2 August B S. haemolyticus + Weak + Yes
S. epidermidis strain ATCC 12228Normalization bands
6
2
2
3 6
7
8
2
2
2
4
5
40 50 60 70 80 90
CoNS sepsis in neonates 25
Ch
ap
ter
2
Discussion
CoNS are the most frequently isolated pathogens in NICU patients worldwide. LOS caused by CoNS leads to significantly higher morbidity and mortality (2,24). Because CoNS are part of the normal skin flora, it is hard to ban them from the NICU. The extensive use of antibiotics has made the infecting CoNS resistant. Strains isolated on a NICU often belong to persistent clones (7,11,12,25,26). It is generally thought, therefore, that improved hygiene could counteract crossinfection and thus lower the incidence of CoNS LOS. We found that CoNS sepsis accounted for 66% of all neonatal bloodstream infections and 70% of the bloodstream infections among VLBW infants. Stoll et. al. have demonstrated a proportion of almost 48% for different NICUs in the United States over the year 2002 (6). In other studies proportions range from 35% to 57% (27-30). The studies reporting relatively low CoNS proportions have observed at least 10% fungi, which explains the lower magnitudes. Still, even disregarding the fungi, the incidence of CoNS LOS in our NICU is relatively high. There are various reasons to consider. For one, we transfer many VLBW infants to regional hospitals before the age of 72 hours, i.e., those no longer in need of intensive care treatment. Consequently, the most vulnerable patients stay at our NICU. Then, even though we applied strict definitions of CoNS sepsis, the possibility still exists that some of the isolated CoNS are contaminants. Multivariate analysis revealed that the SGA and prolonged hospitalization were associated with CoNS bloodstream infections in VLBW children. This is plausible, because SGA infants usually have intravascular catheters in place for a longer period in comparison with appropriate-for-gestational-age infants. Catheters are associated with CoNS bloodstream infection (7). Previous studies also showed an association between prolonged hospital stay and CoNS sepsis (31). In our analyses, we assumed that nonsepsis infants did not suffer from a CoNS sepsis after discharge to a regional hospital for further follow-up. Only few patients may have encountered such an infection after discharge because we observed that most CoNS sepsis episodes occurred in the first 2 weeks of NICU admission.PFGE is the most widely used method for molecular typing of CoNS (7,10,25,26). Although it is a useful method for outbreak analysis, it is also generally used for long-term epidemiological studies. However, these studies interpret PFGE data using criteria for outbreak analysis that are described by Tenover et. al. (32). This may lead to underestimation of genetic clustering. Tenover et. al. therefore recommend less strict criteria for long-term epidemiological analysis of CoNS by PFGE. Strains with 5-6 bands difference in PFGE pattern would then still belong to the same genetic cluster. Because PFGE patterns of CoNS consist of 10-12 bands, we propose PFGE is less suitable for long-term epidemiological studies. RFEL patterns, on the other hand, showed more bands (approximately 30) than did PFGE, thus reducing chances of false positive clustering. As RFEL typing of CoNS has not been described before, we validated this technique by identifying the concordance between these 2 techniques. With a significant r value of 74%, these 2 techniques matched quite well. Although the reason for minor differences in clustering remains unknown, extra bands in the RFEL analysis (eg, caused by plasmids) are considered to contribute to these discrepancies.Genetic relatedness of RFEL analysis correlated very well with VITEK 2 species identification. Different species clustered quite well in the genetic dendrogram, except for 3 strains. Although RFEL is not a validated tool for species identification, this discrepancy might be as a result of a false VITEK 2 identification, as VITEK 2 is known to have an accuracy of approximately 90% (33). ITS-PCR patterns could also be well correlated to specific species,
Chapter 226
although for one pattern (D) we could not identify the species using both VITEK 2 and RFEL. Nevertheless, we believe ITS-PCR is an appropriate method for fast identification of different staphylococcal species.With PFGE, Vermont found 33% of the CoNS isolated at our hospital in 1995 to belong to a single genetic cluster (7). In our study we also found 1 cluster comprising 32% of the isolates, again suggesting a major single source of infection. Some small clusters were found as well, suggestive for cross-contamination. As expected, distribution of species shows high proportions of S. epidermidis, S. haemolyticus and S. capitis. Interestingly, a large proportion of both S. haemolyticus and S. capitis belonged to a single cluster, with respectively 6 of the 8 and 5 of the 6 isolates being identical. Although this strongly suggests a limited clonal spread, these isolates were found throughout the year and on all 3 NICU wards. The largest cluster shows a similar pattern. NICU staff themselves may well have been responsible for spreading these isolates. In our study, less than half of the isolates displayed the in vitro biofilm producing phenotype. This is consistent with a recent study by Klingenberg et. al. who found that 51% of the strains in their cohort displayed a biofilm producing phenotype (15). Where S. epidermidis is clearly capable of producing strong biofilms, S. haemolyticus is mainly a weak biofilm producer and most S. capitis isolates do not produce a biofilm at all. This is remarkable, because the latter 2 species were found in the blood of neonates to be clonally related. Biofilm forming potentials are recognized as major virulence factors in CoNS sepsis. Therefore, it would be expected that isolates without this potential would only appear sporadically. If biofilm negative CoNS can still cause clonal infections, it may well be that CoNS causing bloodstream infection do not necessarily originate from the skin. In this respect it strengthens the hypothesis that improved hygiene is not the only factor involved in reducing CoNS infections. CoNS sepsis has been ascribed to CoNS from mucosa, for example the gastrointestinal tract (34). Effective prevention strategies, hence, require further research to identify the source of CoNS in sepsis.The icaA primers we used are based on the conserved sequences of S. aureus, S. epidermidis and S. caprae and may therefore be negative in our S. haemolyticus isolates. We did find a positive correlation between the presence of the icaA gene and biofilm production in S. epidermidis, but not in other species. This reflects the similar finding of Klingenberg et al for the presence of the icaD gene. However, several other studies describe no correlation between ica and biofilm formation (35,36). Although we did not test for biofilm production in different circumstances, for example by adding glucose to the medium, it is remarkable that most of the S. capitis were icaA positive but did not produce biofilm. We feel that the presence of ica is a weak predictor of biofilm formation in CoNS as a group. Antimicrobial resistance among clinical CoNS isolates is a worldwide problem. Although it is not clear if infection is predisposed by antibiotic resistant strains, resistance does complicate eradication therapies. In our study, we found the mecA gene, which codes for methicillin resistance, in 87% of the strains. Others have reported similar proportions (25,26,37). We found resistance to a broad spectrum of antibiotics, but no vancomycin resistance. Seventy-five to 80% of the strains were multiresistant, irrespective of clustering. Expectedly, all S. haemolyticus isolates were multiresistant (38). The high rate of multiresistance found in blood isolates is likely because of the extensive use of antibiotics in our NICU. The relatively low rate of multiresistance in the largest cluster, suggests that multiresistance is not prerequisite for persistence of isolates. In contrast with other authors, we did not find a relation with multiresistance and biofilm production, although a positive mecA PCR was
CoNS sepsis in neonates 27
Ch
ap
ter
2
associated with a positive icaA PCR among S. epidermidis (15). Because the presence of icaA is also associated with biofilm production in this species, this finding may suggest horizontal transfer of mecA and ica genes between bacteria in a biofilm. In summary, 26% percent of all children and 46% of the VLBW children who were in our NICU for more than 72 hours in 2003 had suffered from LOS. Nearly 70% were because of CoNS. SGA and prolonged hospitalization were associated with CoNS sepsis in VLBW infants. A large S. epidermidis clone was identified, and there may also have been several small cases of cross-contamination. Almost all strains were resistant to 1 or more antibiotics. There were significantly less multiresistant isolates in the largest cluster, suggesting multiresistance is not a prerequisite for CoNS infection nor for persistence. Furthermore, ica is only a predictor for the biofilm forming phenotype in S. epidermidis. Finally, RFEL was found to be a useful tool for epidemiological molecular typing of CoNS and the genetic relatedness of CoNS strains as observed by RFEL analysis correlated very well with the species identification by VITEK.
Acknowledgements
The authors are grateful to Brenda Budiharto and for excellent technical assistance with molecular typing, and to Hélène Boelens and Alex van Belkum for kind advice and experimental guidance. We are also grateful to Paul Mulder for statistical advice, to Justine Peeters, Marijana Vujkovic and Koen Janssens for bioinformatical support and to Ko Hagoort for critically reading this manuscript.
Supported by the Sophia Foundation for Medical Research.
Chapter 228
References
1. Sohn AH, Garrett DO, Sinkowitz-Cochran RL, et al. Prevalence of nosocomial infections in neonatal intensive care unit patients: Results from the first national point-prevalence survey. J Pediatr. 2001;139:821-827.
2. Stoll BJ, Hansen NI, Adams-Chapman I, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. Jama. 2004;292:2357-2365.
3. Blot SI, Depuydt P, Annemans L, et al. Clinical and economic outcomes in critically ill patients with nosocomial catheter-related bloodstream infections. Clin Infect Dis. 2005;41:1591-1598.
4. Payne NR, Carpenter JH, Badger GJ, Horbar JD, Rogowski J. Marginal increase in cost and excess length of stay associated with nosocomial bloodstream infections in surviving very low birth weight infants. Pediatrics. 2004;114:348-355.
5. Stoll BJ, Hansen N. Infections in VLBW infants: studies from the NICHD Neonatal Research Network. Semin Perinatol. 2003;27:293-301.
6. Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285-291.
7. Vermont CL, Hartwig NG, Fleer A, et al. Persistence of clones of coagulase-negative staphylococci among premature neonates in neonatal intensive care units: two-center study of bacterial genotyping and patient risk factors. J Clin Microbiol. 1998;36:2485-2490.
8. Pittet D, Simon A, Hugonnet S, et al. Hand hygiene among physicians: performance, beliefs, and perceptions. Ann Intern Med. 2004;141:1-8.
9. Healy CM, Palazzi DL, Edwards MS, Campbell JR, Baker CJ. Features of invasive staphylococcal disease in neonates. Pediatrics. 2004;114:953-961.
10. Boisson K, Thouverez M, Talon D, Bertrand X. Characterisation of coagulase-negative staphylococci isolated from blood infections: incidence, susceptibility to glycopeptides, and molecular epidemiology. Eur J Clin Microbiol Infect Dis. 2002;21:660-665.
11. Villari P, Sarnataro C, Iacuzio L. Molecular epidemiology of Staphylococcus epidermidis in a neonatal intensive care unit over a three-year period. J Clin Microbiol. 2000;38:1740-1746.
12. Monsen T, Karlsson C, Wistrom J. Spread of clones of multidrug-resistant, coagulase-negative staphylococci within a university hospital. Infect Control Hosp Epidemiol. 2005;26:76-80.
13. Paradisi F, Corti G, Messeri D. Antistaphylococcal (MSSA, MRSA, MSSE, MRSE) antibiotics. Med Clin North Am. 2001;85:1-17.
14. von Eiff C, Peters G, Heilmann C. Pathogenesis of infections due to coagulase-negative staphylococci. Lancet Infect Dis. 2002;2:677-685.
15. Klingenberg C, Aarag E, Ronnestad A, et al. Coagulase-negative staphylococcal sepsis in neonates. Association between antibiotic resistance, biofilm formation and the host inflammatory response. Pediatr Infect Dis J. 2005;24:817-822.
16. Murakami K, Minamide W, Wada K, et al. Identification of methicillin-resistant strains of staphylococci by polymerase chain reaction. J Clin Microbiol. 1991;29:2240-2244.
17. van Steenbergen TJ, Colloms SD, Hermans PW, de Graaff J, Plasterk RH. Genomic DNA fingerprinting by restriction fragment end labeling. Proc Natl Acad Sci U S A. 1995;92:5572-5576.
18. Hermans PW, Sluijter M, Hoogenboezem T, et al. Comparative study of five different DNA fingerprint techniques for molecular typing of Streptococcus pneumoniae strains. J Clin Microbiol. 1995;33:1606-1612.
19. Sluijter M, Faden H, de Groot R, et al. Molecular characterization of pneumococcal nasopharynx isolates collected from children during their first 2 years of life. J Clin Microbiol. 1998;36:2248-2253.
20. Shittu A, Lin J, Morrison D, Kolawole D. Identification and molecular characterization of mannitol salt positive, coagulase-negative staphylococci from nasal samples of medical personnel and students. J Med Microbiol. 2006;55:317-324.
21. Christensen GD, Simpson WA, Younger JJ, et al. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985;22:996-1006.
22. Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic-Vlahovic M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods. 2000;40:175-179.
23. Moretro T, Hermansen L, Holck AL, et al. Biofilm formation and the presence of the intercellular adhesion locus ica among staphylococci from food and food processing environments. Appl Environ Microbiol. 2003;69:5648-5655.
24. Bearman GM, Wenzel RP. Bacteremias: a leading cause of death. Arch Med Res. 2005;36:646-659.25. Raimundo O, Heussler H, Bruhn JB, et al. Molecular epidemiology of coagulase-negative staphylococcal
bacteraemia in a newborn intensive care unit. J Hosp Infect. 2002;51:33-42.
CoNS sepsis in neonates 29
Ch
ap
ter
2
26. Krediet TG, Mascini EM, van Rooij E, et al. Molecular epidemiology of coagulase-negative staphylococci causing sepsis in a neonatal intensive care unit over an 11-year period. J Clin Microbiol. 2004;42:992-995.
27. Makhoul IR, Sujov P, Smolkin T, Lusky A, Reichman B. Pathogen-specific early mortality in very low birth weight infants with late-onset sepsis: a national survey. Clin Infect Dis. 2005;40:218-224.
28. Karlowicz MG, Buescher ES, Surka AE. Fulminant late-onset sepsis in a neonatal intensive care unit, 1988-1997, and the impact of avoiding empiric vancomycin therapy. Pediatrics. 2000;106:1387-1390.
29. Isaacs D. A ten year, multicentre study of coagulase negative staphylococcal infections in Australasian neonatal units. Arch Dis Child Fetal Neonatal Ed. 2003;88:F89-93.
30. Pessoa-Silva CL, Miyasaki CH, de Almeida MF, et al. Neonatal late-onset bloodstream infection: attributable mortality, excess of length of stay and risk factors. Eur J Epidemiol. 2001;17:715-720.
31. Stoll BJ, Gordon T, Korones SB, et al. Late-onset sepsis in very low birth weight neonates: a report from the National Institute of Child Health and Human Development Neonatal Research Network. J Pediatr. 1996;129:63-71.
32. Tenover FC, Arbeit RD, Goering RV, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233-2239.
33. Layer F, Ghebremedhin B, Moder KA, Konig W, Konig B. Comparative study using various methods for identification of Staphylococcus species in clinical specimens. J Clin Microbiol. 2006;44:2824-2830.
34. Costa SF, Miceli MH, Anaissie EJ. Mucosa or skin as source of coagulase-negative staphylococcal bacteraemia? Lancet Infect Dis. 2004;4:278-286.
35. Chaieb K, Mahdouani K, Bakhrouf A. Detection of icaA and icaD loci by polymerase chain reaction and biofilm formation by Staphylococcus epidermidis isolated from dialysate and needles in a dialysis unit. J Hosp Infect. 2005;61:225-230.
36. Fitzpatrick F, Humphreys H, Smyth E, Kennedy CA, O’Gara JP. Environmental regulation of biofilm formation in intensive care unit isolates of Staphylococcus epidermidis. J Hosp Infect. 2002;52:212-218.
37. Caierao J, Musskopf M, Superti S, et al. Evaluation of phenotypic methods for methicillin resistance characterization in coagulase-negative staphylococci (CNS). J Med Microbiol. 2004;53:1195-1199.
38. Takeuchi F, Watanabe S, Baba T, et al. Whole-genome sequencing of staphylococcus haemolyticus uncovers the extreme plasticity of its genome and the evolution of human-colonizing staphylococcal species. J Bacteriol. 2005;187:7292-7308.
30
Chapter
3
Vishal HiraMarcel Sluijter
Wil GoessensAlewijn Ott
Ronald de GrootPeter W. M. Hermans
René F. Kornelisse
J Clin Microbiol. 2010;48(11):3876-81
Coagulase-negative staphylococcal skin
carriage among neonatal intensive care unit
personnel: from population
to infection
For this thesis, Figure 1 was added
30
Chapter
3
Vishal HiraMarcel Sluijter
Wil GoessensAlewijn Ott
Ronald de GrootPeter W. M. Hermans
René F. Kornelisse
J Clin Microbiol. 2010;48(11):3876-81
Coagulase-negative staphylococcal skin
carriage among neonatal intensive care unit
personnel: from population
to infection
For this thesis, Figure 1 was added
Chapter 332
Abstract Coagulase-negative staphylococci (CoNS) are a major cause of sepsis in neonatal intensive care units (NICU) worldwide. Infecting strains of these commensal bacteria may originate from NICU personnel. Therefore, we studied the characteristics of CoNS isolates from NICU personnel and compared them to those of isolates from the general population and from sepsis patients. Furthermore, we studied the epidemiological effect on CoNS carriage of NICU personnel after a period of absence. In our study, we isolated CoNS from the thumbs of NICU personnel every 2 weeks during the summer of 2005 and sampled personnel returning from vacation and a control group from the general population. Furthermore, we collected sepsis isolates from this period. Isolates were tested for antibiotic resistance, mecA and icaA carriage, biofilm production, and genetic relatedness. We found that mecA and icaA carriage as well as penicillin, oxacillin, and gentamicin resistance were significantly more prevalent in CoNS strains from NICU personnel than in community isolates. Similar trends were observed when postvacation strains were compared to prevacation strains. Furthermore, genetic analysis showed that 90% of the blood isolates were closely related to strains found on the hands of NICU personnel. Our findings revealed that CoNS carried by NICU personnel differ from those in the general population. Hospital strains are replaced by community CoNS after a period of absence. NICU personnel are a likely cause for the cross-contamination of virulent CoNS that originate from the NICU to patients.
CoNS skin carriage among NICU personnel 33
Ch
ap
ter
3
Introduction Coagulase-negative staphylococci (CoNS) are the most-frequent cause of late-onset sepsis among newborn infants in neonatal intensive care units (NICU) worldwide. Incidences of up to 66% of late-onset sepsis have been reported (1, 2). The high incidence of these infections is due not only to a high rate of invasive procedures in immunocompromised patients but also to the bacterium’s ability to form biofilms (3).The biofilm-forming property of CoNS generally is considered their most important virulence factor. Biofilm formation is mediated by several factors, such as surface proteins and the polysaccharide intercellular adhesin (PIA). PIA is regulated by the ica operon, and the presence of the ica genes has been shown to be a predictor for biofilm formation in Staphylococcus epidermidis (1, 4). Furthermore, we previously showed a strong association between the carriage of icaA and mecA, the gene coding for methicillin resistance.Antibiotic resistance in CoNS, especially against β-lactam antibiotics, has increased over the years. The mecA gene is present in more than 80% of the CoNS late-onset sepsis isolates (1). The high rate of antibiotic resistance and their biofilm-forming capacities probably enable CoNS to persist in the intensive care environment by giving them a selective advantage over other more-susceptible species.Since CoNS are commensal skin bacteria, it is generally hypothesized that infecting strains originate from NICU personnel. This theory is supported by the fact that NICU personnel carry CoNS that have characteristics similar to those of bloodstream isolates, like high antibiotic resistance. It has been shown previously that new graduate NICU nurses acquire antibiotic-resistant staphylococci over time (5). It is, however, unknown if this colonization persists after a period of absence from the NICU. It also is unknown to what extent CoNS carried by personnel differ from community strains. Since this information can give more insight into the origins of infecting CoNS strains and the dynamics of CoNS carriage, we studied different characteristics, i.e., antibiotic resistance and biofilm-forming properties of CoNS isolated from NICU personnel and community strains. Furthermore, we studied the effect of a period of absence from the NICU on the CoNS carriage of NICU personnel to see if CoNS are replaced. Finally, we compared these isolates to NICU sepsis blood isolates collected in the same period to see if NICU personnel carry the infecting strains. Materials & methods Subjects and setting This study was performed from June to September 2005 at the NICU of Erasmus MC-Sophia Children’s Hospital, Rotterdam, The Netherlands. This NICU consists of three wards with nine level III beds each. All permanently attached doctors and nurses of the NICU were eligible for inclusion. Gender, age, percentage of full-time equivalent (FTE; calculated by dividing the number of hours worked by the number of hours representing full-time employment), first date of employment, antibiotic use in the past 6 months, and vacation plans were recorded at inclusion. If a subject had gone on vacation, upon return to the NICU he or she was asked for the location of the vacation and antibiotic use during vacation. The control group from which the community strains were acquired consisted of subjects from the general population. They were volunteers at a central location of the nonmedical setting of the Erasmus University of Rotterdam. During 3 days in September 2005, passers-by were asked
Chapter 334
to provide samples and to fill in a questionnaire recording their gender, age, postal code, faculty, function, and antibiotic use in the past 6 months.All subjects signed a written consent form. This study was approved by the Medical Ethical Committee of Erasmus University Medical Center, Rotterdam, The Netherlands.
Study design and samples We performed a longitudinal study of the skin carriage of CoNS among NICU personnel. All included subjects were sampled once every 2 weeks during the sample period. When a subject had gone on vacation, a sample was taken immediately after return to the NICU (postvacation sample). Postvacation samples that were taken after entrance to the NICU were excluded. Control subjects were sampled only once. Postvacation samples were compared to the last regular 2-week sample before the vacation (prevacation sample).To remove transient flora, the subjects washed their hands with Palmolive Naturals liquid hand wash with almond milk (Colgate-Palmolive Nederland BV, Weesp, The Netherlands) for at least 30 s and dried their hands with a clean paper towel. Samples were obtained from the thumb of their dominant hand on a phenol-manitol agar plate (5% NaCl). Plates were incubated at 37°C for 2 days and subsequently at room temperature for 5 days. A maximum of three visually different colonies were picked and regrown on tryptic soy agar plates (Figure 1). For control samples from the general population, only one colony was picked. All colonies were tested for catalase and the absence of coagulase activity. Catalase-negative and coagulase-positive strains were excluded. CoNS isolates were stored in glycerol-containing liquid medium at −80°C until further analysis. For comparison to clinical isolates, all CoNS sepsis isolates from the study period were retrieved from the microbiology laboratory. A CoNS sepsis isolate was defined as described before (1).
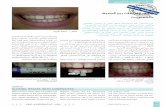
Bacterial analysis Bacterial DNA was isolated using the cetyl trimethylammonium bromide purification method as described before (6). We performed a multiplex PCR detecting the Staphylococcus aureus-specific nuc gene, the mecA gene, the icaA gene, and the staphylococcal 16S RNA based on the multiplex PCR designed by Zhang et al. (7). 16S RNA-negative and nuc-positive samples were excluded from the study. Species identification was done by internal transcribed spacer (ITS) PCR as described before (1). Unknown ITS PCR patterns were identified with Vitek 2 (bioMérieux, Marcy l’Etoile, France). DNA fingerprinting by restriction fragment end labeling (RFEL) was performed as previously described (1). Strains with at least 88% genetic similarity were considered genetically related. When a subject showed identical isolates by RFEL at one time point, only one of these isolates was included for further analysis. Biofilm
Figure 1. Hand sampling procedure. A. Subjects washed their hands with non-antibacterial soap; B. Subject dried their hands with a clean paper towel; C. Subject pressed their thumb on a PMA plate; D. Afted 7 days of incubation, we picked 3 visually different colonies from the PMA plate for further analysis.
CoNS skin carriage among NICU personnel 35
Ch
ap
ter
3
production analysis also was performed as previously described, with the addition of 1% glucose to the initial growth medium (1). Strains with an optical density at 595 nm (OD595) < 0.30, 0.30 ≤ OD595 ≤ 1.0, and OD595 > 1.0 were defined as biofilm-negative, weak biofilm formers, and strong biofilm formers, respectively. Biofilm production was tested on 50 randomly selected strains of the prevacation, postvacation, and control groups. The blood isolates all were tested. Susceptibility determinations for penicillin, oxacillin, gentamicin, erythromycin, clindamycin, cotrimoxazol, levofloxacillin, rifampin, and vancomycin were performed by the disk diffusion methodology (8) in accordance with the guidelines and criteria of the CLSI (6). Oxacillin resistance was detected by the use of cefoxitin as an indicator antibiotic. Resistance was defined by measuring the zone diameters for the respective antibiotics, as defined by the CLSI (6). Resistance for vancomycin was monitored by growth on a vancomycin screen agar. Screen agar contained a concentration of 6 μg/ml. Intermediate resistance was excluded from the analysis. Multiresistance was defined as resistance for three or more antibiotics. We also calculated the mean number of antibiotics for which each group was resistant.
Statistical analysis Statistics were performed with SPSS software, version 11 (Chicago, IL). The chi-square test was used for the univariate significance testing of categorical variables. Differences between groups in other variables were analyzed by the nonparametric (two-tailed) Mann-Whitney U test. P values of <0.05 were considered significant. Results Characteristics of patients and isolates During the 4-month study period, 69 personnel members were included in the study. Fifty-seven went on vacation in the study period, eight of them twice. General characteristics of the subjects are described in Table 1. Approximately one-third of the regular samples showed no growth. Of the postvacation samples, two (3%) showed no growth. After the exclusion of noneligible isolates due to sampling after entrance to the NICU or non-CoNS growth, 30 individuals who went on vacation were included for analysis. Two went on vacation twice. This resulted in 51 prevacation isolates and 80 postvacation isolates. We included 207 controls, of whom all samples showed bacterial growth. One hundred eighty-six isolates were CoNS. The characteristics of these subjects are shown in Table 1. These characteristics were tested for relations with the determined bacterial characteristics. No statistically significant relations were found between the different groups. We retrieved 29 CoNS blood culture isolates of neonates with a CoNS sepsis during the same sample period. Characteristics of these infants can be found in Table 1. Species identification Species identification was performed on all included specimens. The distribution of different species among the four groups is shown in Figure 2. The prevacation, postvacation, and control groups consisted largely of Staphylococcus epidermidis, Staphylococcus haemolyticus, and Staphylococcus warneri. The blood isolate group consisted of a significantly larger proportion of S. epidermidis than the other groups (P < 0.001). There were no significant differences in species proportions for the other groups.
Chapter 336
Figure 2. Bacterial species distribution among the different study populations.
Table 1. General characteristics of personnel, controls, and patientsParameter Findinga
Personnel (n) 69 Male (%) 19 Age 39 (34 - 44) Years of employment 6.4 (3.8 - 13.1) Nurse (%) 77 FTE >0,60 (%) 74 Antibiotic Usage (%) 19Controls (n) 186 Male (%) 48 Age (year) 21 (19 - 23) Living in Rotterdam (%) 59,7 Student (%) 93,5 Hospital contact in the last 6 months (%) 23,7 Antibiotic use (%) 13Patients (n) 15 Male (%) 47 Gestational age (weeks, days) 29 4/7 (27 - 35 5/7) Birth weight (g) 975 (725 - 1820) Days of admission 32 (11 - 59)aData are expressed as median (interquartile range), unless specified otherwise.
Pre-vacation
36%
14%
32%
18%
Post-vacation
34%
11%
47%
8%
Controls
30%
12%39%
19%
Blood isolates
S. epidermidis
S. haemolyticus
S. warneri
Other
90%
7% 3%
CoNS skin carriage among NICU personnel 37
Ch
ap
ter
3
Antibiotic resistance and biofilm formation The incidence of mecA and icaA and biofilm-forming ability in the four groups is shown in Figure 3. The presence of both mecA and icaA is highest in the blood isolate group, followed by the prevacation, postvacation, and control groups. Most of these differences are significant (Figure 3). This is in contrast to biofilm formation, which was lowest among the blood isolates and significantly lower than that for both personnel isolates (P < 0.05).Resistance against antibiotics was determined for the prevacation, postvacation, and control specimens (Table 2). The prevacation isolates, which can be regarded as the normal NICU personnel skin flora, showed a high overall incidence of resistance for most antibiotics. The incidence of antibiotic resistance of the postvacation isolates generally is lower than those of the prevacation strains and higher than those of the controls. This difference between the prevacation and community isolates is significant for oxacillin, gentamicin, and penicillin resistance, as well as for multiresistance (all P < 0.001). For these antibiotics, the postvacation isolates were resistant significantly more often than the community isolates. When we compared the prevacation to the postvacation strains, only gentamicin resistance was significantly higher in the prevacation isolates (P = 0.001). We also calculated the average number of types of resistance per isolate for each group. These numbers differed significantly as well. To determine the relationship between the duration of absence and antibiotic resistance, we analyzed the mean number of days of absence for every antibiotic in the postvacation strains. No association was observed between antibiotic resistance or mecA carriage and a longer period of absence.
Figure 3. Incidence of mecA- and icaA-containing and biofilm-producing strains among the different study populations. *, P < 0.05; **, P < 0.001.
0
10
20
30
40
50
60
70
80
90
100
MecA IcaA Biofilm Production
Blood isolates
Pre-vacation
Post-vacation
Control
****
****
**
**
**
**
%
Chapter 338
Genetic diversity The isolated strains showed highly diverse RFEL patterns (data not shown), although several closely related isolates on the hands of different subjects at different sample time points were found. For 10 subject, we analyzed isolates from all time points. In eight subjects, closely related strains could be found in at least two sample periods. In comparisons of RFEL patterns of all prevacation and postvacation strains, we found that only seven subjects (23%) had strongly related CoNS before and after vacation. Among the blood isolates three large groups of closely related strains, comprising a total of 21 strains (72%), were found (data not shown). We also compared the blood isolates to the prevacation, postvacation, and longitudinal isolates. Of the 29 blood isolates from the sample period, 26 (90%) were closely related to skin isolates of NICU personnel. Figure 4 shows examples of different related and unrelated RFEL patterns. Discussion In this study, we have evaluated various characteristics of CoNS isolated from the hands of NICU personnel. We compared them to community CoNS isolates, studied changes that occur after a period of absence of one to several weeks, and compared the personnel skin isolates to sepsis blood isolates. To our knowledge, this is the first study to show that NICU personnel who leave the NICU for a short (vacation) period carry fewer antibiotic-resistant CoNS than they did before their absence. Two studies have been published in which the staphylococcal colonization of inexperienced (student) nurses were compared to those of experienced (student) nurses (5, 9). Both studies have demonstrated that the experienced group carries more antibiotic-resistant strains than the inexperienced group, and that this
Table 2. Antibiotic resistance proportions (%) of the different groups.
Prevacation Postvacation Control
(n = 51) (n = 80) (n = 186)
Penicillin 80b 68c 51b,c
Oxacillin 55b 43c 8b,c
Gentamicin 32a,b 8a,c 1b,c
Erythromicin 26 26 29
Clindamycin 4 3 4
Co-trimoxazol 9 3 4
Levofloxacin 2 1 1
Rifampicin 2 1 1
Vancomycin 0 0 0
Multiresistance 31b 20c 6b,c
Mean no. of resistanced 2.1a,b 1.5a,c 1.0b,c
aSignificant difference between prevacation and postvacation strains bSignificant difference between prevacation and control strains cSignificant difference between postvacation and control strains dValues represent the number of antibiotics to which each isolate in a group (prevacation, postvacation, or control) is resistant divided by the number of isolates in the group
CoNS skin carriage among NICU personnel 39
Ch
ap
ter
3
difference diminishes after several months. This finding suggests that hospital personnel acquire hospital-associated strains over time. Our study shows that the reverse is also true: hospital personnel can lose their hospital-associated strains after a short period of absence from the hospital environment. Although the difference is only significant for gentamicin resistance, the general trend suggests a change in CoNS colonization. These findings are supported by the results of the RFEL analysis confirming that CoNS colonization changes, as only in 7 out of 30 subjects were prevacation strains and postvacation strains related.The most striking result in our study is the high incidence of blood isolate-related strains on the hands of NICU personnel. Since we only analyzed three morphologically different strains from each thumb instead of all strains, the true incidence is probably even higher. This strongly suggests that virulent CoNS are indeed spread by personnel, as several authors have suggested before (10, 11). As was previously described, appropriate hand hygiene among NICU personnel is important for the reduction of sepsis among neonates (12).Because of restricted antibiotic policies in The Netherlands (13) we expected low antibiotic resistance among community isolates. However, half of the samples were still resistant to penicillin, although this was significantly less than that of the samples from personnel. The significant difference in oxacillin most likely is due to the frequent use of the β-lactam antibiotic flucloxacillin in our NICU. The difference with gentamicin resistance is attributable to aminoglycoside-modifying enzymes, which usually are plasmid or transposon encoded (14). The acquisition and loss of resistance therefore may occur much faster than that with other antibiotics. Interestingly, a quarter of the control strains were resistant to erythromycin, as was the case with the personnel strains.The differences between the personnel and control groups likely can be ascribed to the intensive care environment, where there is a high use of antibiotics. Resistant strains are selected and reside in the unit, where personnel get colonized with these strains. The large
Figure 4. Example of several RFEL gel patterns. (A) S. epidermidis ATCC 12228 control strain; (B) S. epidermidis blood isolate; (C) S. epidermidis prevacation isolate identical to that shown in panel B; (D) S. epidermidis prevacation isolate closely related to that shown in panel B; (E) unrelated S. epidermidis postvacation isolate; (F) unrelated S. warneri postvacation isolate.
Chapter 340
number of mecA-positive strains among the blood isolates suggest an antibiotic selection factor, whereas the large number of icaA-positive strains suggests a biofilm selection factor. It should be noted that a positive association between mecA and icaA carriage has been described (1).Another interesting result is the low number of prevacation strains. One-third of the regular samples (including the prevacation samples) showed no growth, which is a much higher proportion than that for the postvacation and control samples. We believe this difference is due to the extensive use of hand alcohol among NICU personnel, which lowers the number of transferable CoNS. However, the high incidence of antibiotic resistance and mecA- and icaA-positive strains in the prevacation samples also may imply a selection of these strains by the inadequate use of hand alcohol. It is known that low doses of alcohol enhance biofilm formation in CoNS in vitro (15). Therefore, hand rinsing should be done thoroughly as well.Species identification has been done by ITS PCR, which has proven to be a reliable tool (15, 16). Although the species distribution in the prevacation, postvacation, and control groups are comparable, the blood isolate group contains a much larger proportion of S. epidermidis. This is consistent with other studies, where S. epidermidis is described as the most frequently isolated staphylococcal species (1, 4, 17). Surprisingly, the blood isolate group contains significantly fewer biofilm-producing strains as well, even though it does contain significantly more icaA-positive strains. In contrast, previous studies show that S. epidermidis produces more biofilm than other species (4, 18). Our results may be explained by the short period of 4 months in which our strains were isolated. Most isolates in this period belong to three closely related groups, which coincidentally show low biofilm production. Hence, it may as well be that analysis over a longer period shows a positive association between S. epidermidis and biofilm production. A study of the CoNS isolates from our NICU in 2003 does show this association (1).Another notable result is the high incidence of S. warneri on the hands of both NICU personnel and the control group. Two studies from 2007 also describe a high incidence of S. warneri on the hands of NICU nurses (17, 19). Both studies note that previous studies have not described the predominance of S. warneri on the hands of hospital personnel. Cimiotti et al. have suggested that time, geographic region, or specific work settings play an important role (17). We have shown that the latter is not the case: the incidence of S. warneri in our nonmedical control group is as high as that in our medical group. Despite the high incidence of S. warneri in skin samples, there were no S. warneri strains among the blood isolates, suggesting that this species is relatively harmless in neonatal sepsis.There are some flaws in our study that need to be considered. Most importantly, we only analyzed three morphologically different strains from each thumb instead of all strains. Especially in our RFEL analysis, this may have led to an underestimation of recurring strains, as these strains simply may not have been picked. For the comparison of the tested bacterial characteristics, however, we suspect that picking three strains leads to an underestimation in significant differences between prevacation and postvacation strains, as the incidence of these characteristics was higher in most individual cases in the prevacation group.Another flaw concerns our control group. Although we regard our control group as being the general population, this is not entirely correct. Because we took our samples at a university, our control group consists mostly of young adult students. The difference between the control group and the NICU personnel is not only in age but also in gender, as the NICU personnel consists mostly of women. However, we assume these factors have no or limited influence on the studied microbial characteristics.
CoNS skin carriage among NICU personnel 41
Ch
ap
ter
3
In this study, we demonstrated that NICU personnel carry more β-lactam- and gentamicin-resistant, multiresistant, and mecA- and icaA-positive CoNS than community strains. Personnel also carry fewer antibiotic-resistant CoNS after a period of absence. Furthermore, almost all blood isolates from the sample period were related to isolates from the hands of personnel. These findings demonstrate that virulent CoNS are acquired on the NICU, and personnel are likely to be an important cause for cross-contamination with these CoNS. In agreement with many others, we therefore stress the importance of good hand hygiene, as this surely reduces the transfer of CoNS. Acknowledgements We thank all participating nurses and physicians for their friendly cooperation. Furthermore, we thank Alex van Belkum and Willem van Leeuwen for their kind advice and Silvia Estevaõ, Marian Benard, Deborah Horst-Kreft, and Remco van Zundert for their excellent technical assistance.This work was financially supported by the Sophia Foundation for Medical Research.
Chapter 342
References
1. Hira V, Sluijter M, Estevao S, et al. Clinical and molecular epidemiologic characteristics of coagulase-negative staphylococcal bloodstream infections in intensive care neonates. Pediatr Infect Dis J. 2007;26:607-612.
2. Sohn AH, Garrett DO, Sinkowitz-Cochran RL, et al. Prevalence of nosocomial infections in neonatal intensive care unit patients: Results from the first national point-prevalence survey. J Pediatr. 2001;139:821-827.
3. Klingenberg C, Ronnestad A, Anderson AS, et al. Persistent strains of coagulase-negative staphylococci in a neonatal intensive care unit: virulence factors and invasiveness. Clin Microbiol Infect. 2007;13:1100-1111.
4. Klingenberg C, Aarag E, Ronnestad A, et al. Coagulase-negative staphylococcal sepsis in neonates. Association between antibiotic resistance, biofilm formation and the host inflammatory response. Pediatr Infect Dis J. 2005;24:817-822.
5. Cimiotti JP, Wu F, Della-Latta P, Nesin M, Larson E. Emergence of resistant staphylococci on the hands of new graduate nurses. Infect Control Hosp Epidemiol. 2004;25:431-435.
6. CLSI. Performance standards for antimicrobial susceptibility testing: 16th informational supplement, vol. 26. Clinical and Laboratory Standards Institute, Wayne, PA; 2006.
7. Zhang K, Sparling J, Chow BL, et al. New quadriplex PCR assay for detection of methicillin and mupirocin resistance and simultaneous discrimination of Staphylococcus aureus from coagulase-negative staphylococci. J Clin Microbiol. 2004;42:4947-4955.
8. Standards NCfCL. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed.: National Committee for Clinical Laboratory Standards, Wayne, Pa.; 1997.
9. Duncan IB, Collins AM, Neelin EM, Roy TE. Nasal carriage of staphylococcus pyogenes by student nurses. Can Med Assoc J. 1957;77:1001-1009.
10. Lee YL, Cesario T, Lee R, et al. Colonization by Staphylococcus species resistant to methicillin or quinolone on hands of medical personnel in a skilled-nursing facility. Am J Infect Control. 1994;22:346-351.
11. Patrick CH, John JF, Levkoff AH, Atkins LM. Relatedness of strains of methicillin-resistant coagulase-negative Staphylococcus colonizing hospital personnel and producing bacteremias in a neonatal intensive care unit. Pediatr Infect Dis J. 1992;11:935-940.
12. Pessoa-Silva CL, Hugonnet S, Pfister R, et al. Reduction of health care associated infection risk in neonates by successful hand hygiene promotion. Pediatrics. 2007;120:e382-390.
13. Sturm AW. Effects of a restrictive antibiotic policy on clinical efficacy of antibiotics and susceptibility patterns of organisms. Eur J Clin Microbiol Infect Dis. 1990;9:381-389.
14. Mingeot-Leclercq MP, Glupczynski Y, Tulkens PM. Aminoglycosides: activity and resistance. Antimicrob Agents Chemother. 1999;43:727-737.
15. Conlon KM, Humphreys H, O’Gara JP. icaR encodes a transcriptional repressor involved in environmental regulation of ica operon expression and biofilm formation in Staphylococcus epidermidis. J Bacteriol. 2002;184:4400-4408.
16. Shittu A, Lin J, Morrison D, Kolawole D. Identification and molecular characterization of mannitol salt positive, coagulase-negative staphylococci from nasal samples of medical personnel and students. J Med Microbiol. 2006;55:317-324.
17. Cimiotti JP, Haas JP, Della-Latta P, et al. Prevalence and clinical relevance of Staphylococcus warneri in the neonatal intensive care unit. Infect Control Hosp Epidemiol. 2007;28:326-330.
18. de Silva GD, Kantzanou M, Justice A, et al. The ica operon and biofilm production in coagulase-negative Staphylococci associated with carriage and disease in a neonatal intensive care unit. J Clin Microbiol. 2002;40:382-388.
19. Cook HA, Cimiotti JP, Della-Latta P, Saiman L, Larson EL. Antimicrobial resistance patterns of colonizing flora on nurses’ hands in the neonatal intensive care unit. Am J Infect Control. 2007;35:231-236.
Chapter
4
Colonization of neonateswith coagulase-negative
staphylococci
4.1 Colonization dynamics of antibiotic resistant coagulase-
negative staphylococci in neonates
4.2 Early colonization of neonates with antibiotic resistant
coagulase-negative staphylococci is a risk factor
for late-onset sepsis
44
Chapter
4
Colonization of neonateswith coagulase-negative
staphylococci
4.1 Colonization dynamics of antibiotic resistant coagulase-
negative staphylococci in neonates
4.2 Early colonization of neonates with antibiotic resistant
coagulase-negative staphylococci is a risk factor
for late-onset sepsis
Chapter
4.1
Vishal HiraRené F. Kornelisse
Marcel SluijterAlike KamerbeekWil H. GoessensRonald de Groot
Peter W. M. Hermans
J Clin Microbiol. 2013;51:595-597
Colonization dynamicsof antibiotic resistant
coagulase-negativestaphylococci
in neonates
Published in adapted form
46
Chapter
4.1
Vishal HiraRené F. Kornelisse
Marcel SluijterAlike KamerbeekWil H. GoessensRonald de Groot
Peter W. M. Hermans
J Clin Microbiol. 2013;51:595-597
Colonization dynamicsof antibiotic resistant
coagulase-negativestaphylococci
in neonates
Published in adapted form
Chapter 4.148
Abstract
Coagulase-negative staphylococci (CoNS) are the most important cause of late-onset sepsis in neonatal intensive care units (NICU) worldwide. Infecting strains are often antibiotic resistant. In this study, we isolated CoNS from the skin and feces of neonates during their NICU hospitalization. Furthermore, we isolated CoNS from the skin of mothers of these children. We observed that antibiotic resistance in CoNS isolated from the skin of neonates increased during hospitalization, with multidrug resistance increasing from 40% to 91%. In addition, antibiotic resistance rates in CoNS isolated from the gut immediately after birth were high, with multidrug resistance rates varying between 91% and 100%. In particular Staphylococcus haemolyticus displayed an increase in antibiotic resistance over time (25% to 100% for multidrug resistance). Staphylococcus warneri was less frequently antibiotic resistant compared to other species. Based on these data, we conclude that colonization with antibiotic resistant CoNS increases during hospitalization on the NICU and different CoNS species may play a distinct role in these dynamics.
Antibiotic resistant CoNS in neonates 49
Ch
ap
ter
4.1
Introduction
Coagulase-negative staphylococci (CoNS) are the most frequent cause of late-onset sepsis among newborn infants in neonatal intensive care units (NICU) worldwide with incidences up to 66% of all late-onset sepsis in our hospital (1).One of the major concerns is the increasing antibiotic resistance of these bacteria. The mecA gene, for example, is present in over 80% of the CoNS late-onset sepsis isolates in The Netherlands (1). The major virulence characteristic of CoNS is its ability to form biofilms. CoNS attach to intravascular lines, where they form biofilms. Eventually, bacteria detach from the biofilm, giving them the opportunity to infect the bloodstream (2). It has been demonstrated that antibiotic resistance and biofilm formation in CoNS are positively correlated (3).Since CoNS are commensal skin bacteria, it is generally assumed that the infecting bacteria reside on the skin of the children, from where they enter the bloodstream. However, there are also theories that the strains originate from the gut of the children (4). Bloodstream isolates have proven to be frequently antibiotic resistant, similar to CoNS isolates from NICU personnel and from NICU sites. In a previous study it was shown that the majority of CoNS causing sepsis among neonates can be found on the hands of NICU personnel (5). Since there is a low incidence of antibiotic resistant CoNS in the non-medical population, it is generally assumed that neonates are NICU-isolate free at the moment of birth. It is, however, unknown how skin and gut colonization with resistant CoNS develops during NICU hospitalization over time. A better understanding of CoNS colonization dynamics is considered to assist to the development of future preventive strategies, for example improvement of hygienic measures. We therefore studied the colonization dynamics of neonates with CoNS, focusing on the development of antibiotic resistance. We investigated both skin and intestinal colonization, as well as maternal colonization after birth. Materials & methods
Subjects and SettingThis study was performed from mid November 2006 to mid March 2007 at the NICU of Erasmus MC – Sophia Children’s Hospital, Rotterdam, The Netherlands. This NICU consists of three wards with nine level III beds each. All inborn infants with a presumed hospitalization time of at least seven days (gestational age ≤ 30 weeks, birthweight ≤ 1500g or other reason) were included in this study. Children with a final hospitalization time of less than four days were excluded. Parents of all subjects signed a written consent form. This study was approved by the Medical Ethical Committee of Erasmus University Medical Center, Rotterdam.
Study Design and SamplesWe performed a longitudinal study of skin and intestinal carriage of CoNS among neonates and their mothers. All included infants were sampled 24, 48 and 72 hours (+/- 4 hours), 7 days and, if still admitted, 14 and 21 days after birth. Their mothers were sampled only once in the first 3 days, and then after 7, 14 and 21 days after delivery.Skin samples from infants were obtained by gently pressing the bottom of a foot on a phenol-mannitol agar (PMA) plate (5% NaCl). Intestinal samples were obtained by culturing feces
Chapter 4.150
on PMA plate. Mothers first washed their hands with Palmolive Naturals Liquid handwash with Almond Milk (Colgate-Palmolive Nederland BV, Weesp, The Netherlands) for at least 30 seconds to remove transient flora, and dried their hands with a clean paper towel. Their samples were obtained by gently pressing the thumb of their dominant hand on a PMA plate. Plates were incubated at 37°C for two days and subsequently at room temperature for five days. A maximum of three visually different colonies were picked and regrown on tryptic soy agar plates (Figure 1). All colonies were tested for catalase and absence of coagulase activity. Catalase- negative and coagulase-positive strains were excluded. CoNS isolates were stored in glycerol-containing liquid media at -80°C until further analysis.
Figure 1. Sampling and culture of the different sample sites. A: Neonatal skin samples were obtained by pressing the bottom of the foot on PMA agar; B: Neonatal intestinal saples were obtained by collecting feces and culturing them om a PMA plate; C: Maternal skin samples were obtained by pressing the thumb on a PMA plate; D: Afted 7 days of incubation, we picked 3 visually different colonies from the PMA plate for further analysis.
Bacterial AnalysisWe isolated bacterial DNA using the CTAB purification method as described before (1). We performed a multiplex PCR detecting the S. aureus specific nuc gene, the mecA gene, the icaA gene, and the staphylococcal gene encoding 16S RNA using protocol designed by Zhang et al (6). 16S RNA negative and nuc positive samples were excluded from the study. Species identification was done by ITS PCR as described by Shittu et. al. (7). Susceptibility determinations for penicillin, oxacillin, gentamicin, erythromycin, clindamycin, co-trimoxazol, levofloxacin, rifampicin and vancomycin were performed by the disk diffusion methodology (8) according to the guidelines and criteria of the Clinical and Laboratory Standards Institute (9). Oxacillin resistance was detected by using of cefoxitin as indicator antibiotic. Resistance was defined by measuring the zone diameters for the respective antibiotics, as defined by the CLSI (9). Resistance for vancomycin was monitored by growth on vancomycin screen agar. Screen agar contained a concentration of vancomycin of 6 μg/ml. Intermediate resistant isolates were regarded as resistant. If more than one isolate at a certain sample site and timepoint showed identical antibiograms, only one isolate was included in the final analysis. Multidrug resistance was defined as resistance for three or more antibiotics.
Statistical Analysis Statistical analyses were performed with the Statistical Package of Social Sciences (SPSS) software, version 17 (Chicago, Illinois, USA). The Chi-square test was used for univariate significance testing of categorical variables, unless counts were <5. In that case, Fisher’s Exact test was used. With Linear-by-Linear Association, assessment of changes at a sample site over time was performed. Differences between groups in other variables were analyzed by the nonparametric (two-tailed) Mann-Whitney U test. In view of the multiple tests
Antibiotic resistant CoNS in neonates 51
Ch
ap
ter
4.1
performed, we set the limit of significance at P=0.01 (two-sided), instead of the conventional P=0.05.
Results
During the five-month study period, 41 infants were included in the study. One of these children was excluded after being discharged within 72 hours. Four pairs of twins were included. General characteristics for these children are summarized in Table 1. Seventeen children were hospitalized for one week, 11 for two weeks and six for at least three weeks. Thirty-seven children received one gift of penicillin and gentamicin directly postpartum. Five patients died during hospitalization, their mean survival was 24 days.
The skin was CoNS culture negative in nine (23%) children at 24 hours after birth. In one (3%) of these children, the CoNS culture was also negative at 48 hours. In one child CoNS culture was positive at 24 hours but negative at 48 hours. For all other time points, CoNS cultures were positive for all children. Defecation did not occur every day in the first three days after birth. In this period, we obtained one sample from 13 (33%) children, two samples from 14 (35%) children and three samples from five (13%) children. Eight (20%) children did not have defecation in the first 72 hours after birth. Intestinal samples were available for all other timepoints. The first maternal sample was negative for CoNS in four cases (11%). Maternal samples at other timepoints were all positive for CoNS. After exclusion of all non-eligible isolates, a total of 559 isolates were analyzed. The number of isolates per timepoint is shown in Table 2. Antibiotic resistance rates throughout the hospitalization period are shown for each antibiotic, as well as for multidrug resistance and the presence of mecA (Figure 2). Penicillin, gentamicin and cefoxitin resistance, as well as multidrug resistance and mecA carriage of CoNS from skin and feces from neonates were high (40-100%) during the entire sample period. Antibiotic resistance and mecA carriage were consistently low (0 – 29%) in maternal isolates, except for penicillin resistance (17-65%) and erythromycin resistance (27-50%). None of the isolates was resistant to vancomycin.Skin isolates showed significantly increasing antibiotic resistance over time for levofloxaxin, gentamicin, cefoxitin, as well as increasing mecA carriage and multidrug resistance (all P<0.001). Intestinal and maternal isolates did not show any significant changes over time in antibiotic resistance, although penicillin resistance in maternal isolates showed a clear trend in increase (P=0.028).
Table 1. General characteristics of included neonates (n = 40)
Birthweight (grams) (range; sd) 1185 (530-1650; 291)Gestational age (weeks) (range; sd) 28 4/7 (25-32 2/7; 1 6/7)Male (%) 65Ceasarian section (%) 33Postpartum antibiotics1 (%) 93Antibiotics during admission2 (%) 80Hospitalisation (days) (range; sd) 21.6 (5-92; 19.4)Mortality (%) 13
Data are expressed as mean, unless specified otherwise. sd: standard deviation1Postpartum antibiotics consisted of one gift of penicillin and gentamicin directly postpartum.2Antibiotics were given on indication (e.g. suspected or proven nosocomial infection). The choice of antibiotics depended on the indication.
Chapter 4.152
0
20
40
60
80
100
24 48 72 7 14 21
%
Gentamicin
Hours Days
*$
%$
*$
%$
%$
%
0
20
40
60
80
100
24 48 72 7 14 21
%
Penicillin
Hours Days
%$
%$ $
%$
%$
0
20
40
60
80
100
24 48 72 7 14 21
%
Levofloxacin
Hours Days
%$
0
20
40
60
80
100
24 48 72 7 14 21
%
Rifampicin
Hours Days
0
20
40
60
80
100
24 48 72 7 14 21
%
Cotrimoxazol
Hours Days
0
20
40
60
80
100
24 48 72 7 14 21
%
Erythromycin
Hours Days
0
20
40
60
80
100
24 48 72 7 14 21
%
Clindamycin
Hours Days
0
20
40
60
80
100
24 48 72 7 14 21
%
Cefoxitin%$
%$
*$
%$
%$ $
Hours Days
0
20
40
60
80
100
24 48 72 7 14 21
%
Multiresistance
Hours Days
%$
%$
*%$
%$
%$
%$
0
20
40
60
80
100
24 48 72 7 14 21
%
mecA
Hours Days
*%$
%$
*$
%$
%$
%$
Figure 2. Rates of resistant isolates (%) for each tested antibiotic at different time points. The solid line (—) depicts neonatal skin isolates, the dotted line (•••) depicts neonatal intestine isolates and the dashed line (---) depicts maternal isolates. Statistically significant differences are marked by * for difference between skin and gut isolates, by % for difference between skin and maternal isolates, and by $ for difference between gut and maternal isolates.
Antibiotic resistant CoNS in neonates 53
Ch
ap
ter
4.1
When comparing isolates at the three different sample sites, we observed that CoNS isolates from feces were generally more antibiotic resistant and mecA positive than other isolates. The difference in antibiotic resistance between CoNS isolates from feces and skin isolates is especially notable after 72 hours for gentamicin resistance (95% vs 58%, P<0.001), erythromycin resistance (76% vs 46%, P=0.003), cefoxitin resistance (91% vs 67%, P=0.006), multidrug resistance (98% vs 61%, P<0.001) and mecA carriage (98% vs 75%, P=0.001). At most timepoints, there was significantly less penicillin, gentamicin and cefoxitin resistance as well as mecA carriage and multidrug resistance in maternal isolates compared to both skin and intestinal isolates. S. epidermidis was the most prevalent species among skin (33%) and intestinal (53%) isolates, whereas S. warneri was the most prevalent species among maternal isolates (35%) (Figure 3). Compared to intestinal isolates, skin isolates were significantly less often S. epidermidis (33% vs. 53%, P<0.001) and more often S. warneri (23% vs. 9%, P=0.002) or other CoNS species (24% vs. 6%, P<0.001). There was a significant increase in S. haemolyticus over time among skin isolates (9% at T=24 h to 25% at T=21 days, P=0.002), with a non-significant decrease of S. epidermidis (38% at T=24 h to 8% at T=21 days, P=0.024) and S. warneri (30% at T=24 h to 0% at T=21 days, P=0.049) (Figure 4). Species among other isolates did not change significantly over time. Compared to other species, S. warneri isolates were significantly less levofloxacin (3% vs. 32%, P<0.001), co-trimoxazole (3% vs. 24%, P<0.001) and erythromycin (34% vs 58%, P<0.001) resistant, as well as less multidrug iresistant (52% vs. 67%, P=0.004). Levofloxacin resistance (P=0.004), gentamicin resistance (P=0.002), cefoxitin resistance (P<0.001), mecA carriage (P<0.001) and multidrug resistance (P=0.004) increased over time among S. haemolyticus skin isolates (Figure 5).
Table 2. Number of included isolates at each timepoint
# of Isolates Timepoint Skin Intestinal Maternal
24 56 8 13Hours 48 73 13 16
72 76 43 67 71 47 45
Days 14 32 12 16 21 14 6 12
Skin Intestine
Mother
S. epidermidis
S. haemolyticus
S. warneri
Other
33%
19%24%
24%
54%
31%
9%6%
29%
11%
36%
24%
Figure 3. Bacterial species distribution among the different study groups.
Chapter 4.154
0%
10%
20%
30%
40%
50%
60%
70%
80%
90%
100%
24 48 72 7 14 21
Other
S. warneri
S. haemolyticus
S. epidermidis
Hours Days
Figure 4. Bacterial species distribution among the skin isolates over time.
0%
10%
20%
30%
40%
50%
60%
70%
80%
90%
100%
24 48 72 7 14 21
Levofloxacin
Gentamicin
Cefoxitin
MecA
Multiresistance
Hours Days
Figure 5. Levofloxacin resistance, gentamicin resistance, cefoxitin resistance, mecA carriage and multidrug resistance among S. haemolyticus skin isolates over time.
Antibiotic resistant CoNS in neonates 55
Ch
ap
ter
4.1
Discussion
In this study we investigated the development of antibiotic resistance dynamics of CoNS in infants during their stay on a NICU. To our knowledge this is the first study to show the dynamics of antibiotic resistance in coagulase-negative staphylococci on the skin and in the gut of neonates. As expected, antibiotic resistance in CoNS on the skin of neonates is low right after birth, but increases rapidly in the first week of hospitalization, especially for the antibiotics which are more often used at the NICU, such as gentamicin and β-lactam antibiotics. Our data show that children are not colonized with NICU related strains at birth. This is supported by the finding that resistance of maternal isolates is low for all antibiotics except for penicillin. The higher resistance for penicillin is consistent with previous findings showing 50% of the CoNS in the general population is penicillin resistant (5). Interestingly, penicillin resistance is constantly high throughout hospitalizaion and does not rise significantly. Almost all children received antibiotics, consisting of penicillin and gentamicin, at birth. This may explain why almost all CoNS on the NICU are penicillin resistant. It has been shown that staphylococci are among the first gut colonizers (10). A striking finding in our study is the high antibiotic resistance among the fecal isolates. It is most widely accepted that the skin is the primary source of infecting CoNS. However in 1982 Wade et. al. have already proposed that gut may be the primary source (11). The gut theory proposes that after mucosal damage, CoNS translocate through mesenteric lymph nodes, eventually ending in the blood stream, resulting in a bacteremia (12). In the past 30 years, several studies have shown that CoNS from CoNS bacteremia in different patient populations could be found on mucosa (13-15). In our study, antibiotic resistance in fecal isolates is very high from the beginning, assuming that antibiotics may penetrate well into gut mucosa. However, gut CoNS consisted of other species than skin CoNS. Most notably, S. warneri, which showed to be the least resistant species, was significantly less prevalent among gut CoNS. It is unknown whether S. warneri is cleared from the gut by antibiotics or the gut is unhealthy environment for S. warneri. As suggested before, S. warneri is probably a relatively harmless species in neonatal sepsis (5). On the other hand, we have shown that S. haemolyticus is a good gut colonizer. During hospitalization, S. haemolyticus also becomes more prevalent on the skin as well as becoming increasingly antibiotic resistant. Several studies have shown that S. haemolyticus is an important species in neonatal CoNS sepsis (16-18). Further studies on the role of S. haemolyticus in intestinal colonization and sepsis of neonates are therefore necessary. There are several potential flaws in our study. First of all, we only sampled the bottom of one foot of the children and no other skin parts. Our choice for sampling the bottom of a foot was made out of practicality. The foot of such small infants is easily accessible in the incubator and therefore the discomfort for the children is minimal. We doubt that microbial colonization of feet and other exposed skin, for example hands, differ at this age, as there is no weight pressure on the feet yet. However, it cannot be ruled out that differences in skin composition, for example because of a different pattern of sweat glands, cause a difference in microbial colonization. Another possible flaw is the fact that we only picked three colonies from each cultured sample and did not analyze them all. In theory, this could result in either an under- or overestimation of resistant isolates at each time point. As there were usually no more than three different strains visible in each sample, we assume that analysis of all strains would result in a limited change in the percentage of resistant isolates.
Chapter 4.156
In summary, we showed that neonates are colonized with resistant CoNS right after birth, especially in the gut. Resistant skin isolates, especially S. haemolyticus, become more prevalent during hospitalization on the NICU, while prevalence of the antibiotic sensitive S. warneri decreases, implying important resistance differences among CoNS species. Our data contribute to an increased understanding of CoNS colonization dynamics and possibly preventive strategies, for example by stimulation of less virulent CoNS species.
Acknowledgements
The authors would like to thank all participating parents and infants for their friendly cooperation. Furthermore, the authors would like to thank Wim Hop for statistical advice and Joyce van der Weijde and Remco van Zundert for their excellent technical assistance.
Antibiotic resistant CoNS in neonates 57
Ch
ap
ter
4.1
References
1. Hira V, Sluijter M, Estevao S, et al. Clinical and molecular epidemiologic characteristics of coagulase-negative staphylococcal bloodstream infections in intensive care neonates. Pediatr Infect Dis J. 2007;26:607-612.
2. von Eiff C, Peters G, Heilmann C. Pathogenesis of infections due to coagulase-negative staphylococci. Lancet Infect Dis. 2002;2:677-685.
3. Klingenberg C, Aarag E, Ronnestad A, et al. Coagulase-negative staphylococcal sepsis in neonates. Association between antibiotic resistance, biofilm formation and the host inflammatory response. Pediatr Infect Dis J. 2005;24:817-822.
4. Costa SF, Miceli MH, Anaissie EJ. Mucosa or skin as source of coagulase-negative staphylococcal bacteraemia? Lancet Infect Dis. 2004;4:278-286.
5. Hira V, Sluijter M, Goessens WH, et al. Coagulase-negative staphylococcal skin carriage among neonatal intensive care unit personnel: from population to infection. J Clin Microbiol. 2010;48:3876-3881.
6. Zhang K, Sparling J, Chow BL, et al. New quadriplex PCR assay for detection of methicillin and mupirocin resistance and simultaneous discrimination of Staphylococcus aureus from coagulase-negative staphylococci. J Clin Microbiol. 2004;42:4947-4955.
7. Shittu A, Lin J, Morrison D, Kolawole D. Identification and molecular characterization of mannitol salt positive, coagulase-negative staphylococci from nasal samples of medical personnel and students. J Med Microbiol. 2006;55:317-324.
8. Standards NCfCL. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 4th ed National Committee for Clinical Laboratory Standards, Wayne, PA 1997.
9. Clinical and Laboratory Standards Institute W, PA. Performance standards for antimicrobial susceptibility testing: 16th informational supplement, vol. 26. 2006.
10. Adlerberth I, Lindberg E, Aberg N, et al. Reduced enterobacterial and increased staphylococcal colonization of the infantile bowel: an effect of hygienic lifestyle? Pediatr Res. 2006;59:96-101.
11. Wade JC, Schimpff SC, Newman KA, Wiernik PH. Staphylococcus epidermidis: an increasing cause of infection in patients with granulocytopenia. Ann Intern Med. 1982;97:503-508.
12. Costa SF, Barone AA, Miceli MH, et al. Colonization and molecular epidemiology of coagulase-negative Staphylococcal bacteremia in cancer patients: a pilot study. Am J Infect Control. 2006;34:36-40.
13. Eastick K, Leeming JP, Bennett D, Millar MR. Reservoirs of coagulase negative staphylococci in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1996;74:F99-104.
14. Herwaldt LA, Hollis RJ, Boyken LD, Pfaller MA. Molecular epidemiology of coagulase-negative staphylococci isolated from immunocompromised patients. Infect Control Hosp Epidemiol. 1992;13:86-92.
15. Matsuda J, Hirakata Y, Iori F, et al. Genetic relationship between blood and nonblood isolates from bacteremic patients determined by pulsed-field gel electrophoresis. J Clin Microbiol. 1998;36:3081-3084.
16. Foka A, Chini V, Petinaki E, et al. Clonality of slime-producing methicillin-resistant coagulase-negative staphylococci disseminated in the neonatal intensive care unit of a university hospital. Clin Microbiol Infect. 2006;12:1230-1233.
17. Klingenberg C, Ronnestad A, Anderson AS, et al. Persistent strains of coagulase-negative staphylococci in a neonatal intensive care unit: virulence factors and invasiveness. Clin Microbiol Infect. 2007;13:1100-1111.
18. Low DE, Schmidt BK, Kirpalani HM, et al. An endemic strain of Staphylococcus haemolyticus colonizing and causing bacteremia in neonatal intensive care unit patients. Pediatrics. 1992;89:696-700.
Chapter
4.2
Vishal HiraMarcel Sluijter
Wil H. GoessensRonald de Groot
Peter W. M. HermansRené F. Kornelisse
Submitted
Early colonization of neonates with antibiotic
resistant coagulase-negative staphylococci
is a risk factor for late-onset sepsis
58
Chapter
4.2
Vishal HiraMarcel Sluijter
Wil H. GoessensRonald de Groot
Peter W. M. HermansRené F. Kornelisse
Submitted
Early colonization of neonates with antibiotic
resistant coagulase-negative staphylococci
is a risk factor for late-onset sepsis
Chapter 4.260
Abstract
Introduction Coagulase-negative staphylococci (CoNS) are a major cause of neonatal late-onset sepsis worldwide. As blood isolates are frequently resistant against antibiotics, we studied antibiotic resistance in CoNS on the skin of neonates within 72 hours after birth.
Methods We sampled the skin of neonates at 24, 48 and 72 hours after birth and compared antibiotic resistance profiles of isolates from children who did and those who did not develop CoNS sepsis during NICU hospitalization.
Results Isolates from neonates who developed CoNS sepsis were significantly more often multiresistant and resistant to gentamicin than isolates from neonates who did not develop CoNS sepsis.
Conclusion Carriage of antibiotic resistant CoNS soon after birth is a risk factor for development of CoNS sepsis.
Antibiotic resistant CoNS and LOS 61
Ch
ap
ter
4.2
Introduction Coagulase-negative staphylococci (CoNS) are the most frequent cause of late-onset sepsis, defined as sepsis after 72 hours after birth, among newborn infants in neonatal intensive care units (NICU) worldwide with incidences up to 66% in our hospital (1). There is an increasing antibiotic resistance in these bacteria, especially against β-lactam antibiotics. The mecA gene, coding for methicillin resistance, is present in over 80% of the CoNS late-onset sepsis isolates (1). The ability to form biofilms is thought to be a major virulence characteristic of CoNS. The most common theory for infection is that CoNS attach to intravascular catheters, where they form a biofilm. The biofilm protects the bacteria from hostile factors, like antibiotics. Eventually, the biofilm ruptures and releases bacteria that subsequently infect the bloodstream (2). Antibiotic resistance and biofilm formation in CoNS are positively correlated (3). Several risk factors for infection in neonates have been identified, the most important ones being very low birth weight, low gestational age and, obviously, the presence of intravascular devices. Blood isolates from neonates with CoNS sepsis are frequently antibiotic resistant and up to 90% carry the mecA gene (1, 4, 5). This is in contrast with CoNS on the hands of hospital personnel, of which approximately 50% contains the mecA gene (6). It has been shown that neonates are colonized with antibiotic resistant bacteria as well. It is, however, unknown whether there is a difference in antibiotic resistance between CoNS of neonates who develop CoNS sepsis and those who do not. We have therefore studied the antibiotic resistance rates in CoNS cultured from the skin of neonates at the NICU in the first 72 hours after birth, and analyzed whether late-onset sepsis, defined as sepsis occurring later than 72 hours after birth, was correlated with the occurrence of antibiotic resistance in the first three days of life. Secondly, we compared the resistance patterns of the CoNS from children who did and those who did not suffer from CoNS sepsis.
Materials & methods
Subjects and SettingThis study was performed from June 2005 to September 2005 at the NICU of Erasmus MC – Sophia Children’s Hospital, Rotterdam, The Netherlands. The NICU consists of eighteen level III beds. We included all inborn infants with a predicted hospitalization time of at least seven days (gestational age ≤ 30 weeks, birthweight ≤ 1500g or other reason). We excluded those with a final hospitalization time of less than four days. We obtained written consent from the parents of all subjects. This study was approved by the Medical Ethical Committee of Erasmus University Medical Center, Rotterdam.
Study Design and SamplesThis study was part of a larger study in which we performed longitudinal follow up of skin and intestinal carriage of CoNS among neonates and their mothers (Chapter 5). For this study, all infants were sampled 24, 48 and 72 hours (+/- 4 hours) after birth. Their mothers were also sampled once during the first 3 days after birth. Late-onset sepsis was defined as described by Stoll et al and Klingenberg et al and required clinical signs of sepsis after 72 hours of age, one or more positive blood cultures for only CoNS and a raised CRP (>10 mg/L) within 2 days of blood culture (3, 7).
Chapter 4.262
We obtained skin samples from infants by gently pressing the bottom of a foot on a phenol-manitol agar (PMA) plate (5% NaCl). We also obtained intestinal samples by culturing feces on PMA plate. Before sampling, mothers washed their hands with Palmolive Naturals Liquid handwash with Almond Milk (Colgate-Palmolive Nederland BV, Weesp, The Netherlands) for at least 30 seconds to remove transient flora, and dried their hands with a clean paper towel. Their samples were obtained by gently pressing the thumb of their dominant hand on a PMA plate. Plates were incubated at 37°C for two days and subsequently at room temperature for five days. A maximum of three visually different colonies were picked and regrown on tryptic soy agar plates. All colonies were tested for catalase and absence of coagulase activity. We excluded all catalase negative and coagulase-positive strains. CoNS isolates were stored in glycerol-containing liquid media at -80°C until further analysis.
Bacterial AnalysisWe isolated bacterial DNA using the CTAB purification method as described before (1). A multiplex PCR based on the method of Zhang et al (8), detecting the S. aureus specific nuc gene, the mecA gene, the icaA gene, and the staphylococcal 16S RNA, was performed subsequently. 16S RNA negative and nuc positive samples were excluded from the study. Susceptibility analysis for penicillin, gentamycin, erythromycin, clindamycin, co-trimoxazol, levofloxacillin, rifampicin, cefoxitin and vancomycin was performed by the disk diffusion methodology (9) in accordance with the guidelines and criteria of the Clinical and Laboratory Standards Institute (CLSI) (10). Oxacillin resistance was detected by use of cefoxitin as indicator antibiotic. Resistance was defined by measuring the zone diameters for the respective antibiotics, as defined by the CLSI (10). Resistance for vancomycin was monitored by growth on vancomycin screen agar. Screen agar contained a concentration of 6 μg/ml. Intermediate resistance was regarded as resistant. Multiresistance was defined as resistance for three or more different antibiotics.
Statistical Analysis Statistical analysis was performed with the Statistical Package of Social Sciences (SPSS) software, version 17 (Chicago, Illinois, USA). The Fisher’s exact test was used for univariate significance testing of categorical variables. Differences between groups in other variables were analyzed by the independent Student’s t-test. P values of <0.05 were considered significant.
Results
Forty-one infants were included in the study during the five-month study period. One child was discharged within 72 hours and excluded from our study. Fifteen of these children developed late-onset CoNS sepsis. The general characteristics of these children are shown in Table 1. After exclusion of all non-eligible isolates, 106 skin isolates were analyzed, 54 from patients who did not suffer from CoNS sepsis, 52 from patients who did. Resistance for different types of antibiotics among the isolates from children with and without sepsis is shown in Table 2. Strikingly, children who do not develop CoNS sepsis are colonized with significantly less multiresistant isolates than children who develop CoNS sepsis. Between the two groups, there was no significant difference in birth weight, gestational
Antibiotic resistant CoNS and LOS 63
Ch
ap
ter
4.2
age, administration of antibiotics at birth, or antibiotic resistant CoNS in the gut or the mother. Children who suffered from CoNS sepsis did have a significantly longer mean NICU hospitalization duration than those without CoNS sepsis (32 vs 14 days, P=0.005).
Discussion
In this study we have investigated the antibiotic resistance characteristics of CoNS isolated from neonates in the first 72 hours after birth, and compared neonates who developed late-onset CoNS sepsis during their stay on the NICU with those who did not. We observed that children who developed late-onset CoNS sepsis carried more gentamicin resistant and multiresistant CoNS strains in the first 72 hours after birth than neonates who did not develop CoNS sepsis. One of the possible reasons for the difference in the presence of antibiotic resistant strains could be the use of postpartum treatment with antibiotics. In our hospital, the combination of penicillin and gentamicin are given when indicated at birth. Although there was no significant difference in administration of postpartum
Table 1. General characteristics of neonates who did and did not suffer from CoNS sepsis
Sepsis No sepsis P-value
Number of patients 15 25
Birthweight (grams) 1138 (530-1650; 314) 1213 (770-1630; 279) 0.433
Gestational age (weeks) 27 6/7 (25 1/7-29 6/7; 1.5) 29 (25-32 2/7; 2.1) 0.066
Male (%) 80 56 0.177
Ceasarian section (%) 29 39 0.724
Hospitalisation (days) 33.4 (10-92; 26.2) 13.8 (4-36; 8.9) 0.002
Deceased (%) 13 12 1.000
Data are expressed as mean (range; standard deviation), unless specified otherwise.
Table 2. Percentage of resistant strains among isolates from non-sepsis and sepsis patients
Sepsis No sepsis P-value
Number of strains 52 54
Penicillin 98 87 0.060
Levofloxacin 15 15 1.000
Gentamicin 60 41 0.012
Rifampicin 8 2 0.201
Co-trimoxazol 10 17 0.392
Erythromycin 50 33 0.114
Clindamycin 10 7 0.739
Cefoxitin 62 50 0.248
Vancomycin 0 0 -
Multiresistance 60 41 0.012
mecA positive 69 61 0.426
Chapter 4.264
antibiotics between the two groups, patients who did not receive postpartum antibiotics did not develop CoNS sepsis. It is possible that the administration of postpartum antibiotics promotes the selection of resistant strains and thus enhances the chance of CoNS sepsis. However, postpartum antibiotics are given on indication. Therefore children who do not receive them are presumably in a better clinical condition or do not have risk factors compared to other children. The only significant difference in general characteristics of the two patient groups was the length of hospitalization. It is known that CoNS sepsis leads to longer hospitalization, but long hospitalization is also a risk factor for CoNS sepsis(1, 7). However, the difference in hospitalization length cannot explain the difference in colonization with multiresistant CoNS in the first few days after birth. Since other obvious risk factors such as birth weight and gestational age were equal in both sepsis and non-sepsis groups, the difference may be explained by other factors such as differences in host defense. In in vivo mice studies it has been shown that T-cell deficient mice are more susceptible towards S. epidermidis biofilm infections (11). Carriage of resistant bacteria shortly after birth may also be due to a distinct genetic make-up. It is suggested for example, that the MBL haplotype A is associated with nasal carriage of S. aureus (12). However, the mechanisms of host-pathogen interactions are still poorly understood and it is unlikely that host factors that predispose for colonization of more resistant CoNS will be found in the near future. In summary, we observed that carriage of antibiotic resistant CoNS forms a risk factor for the development of late-onset CoNS sepsis. It remains, however, unclear why some children carry more antibiotic strains than others, and whether the presence of antibiotic strains has a direct role in the development of CoNS sepsis. It is, therefore, necessary to perform additional studies to investigate the role of the neonatal immune system and the possible association between antibiotic resistance and virulence. A better understanding of these issues may open possibilities for future guidance and prevention of CoNS sepsis.
Antibiotic resistant CoNS and LOS 65
Ch
ap
ter
4.2
References
1. Hira V, Sluijter M, Estevao S, et al. Clinical and molecular epidemiologic characteristics of coagulase-negative staphylococcal bloodstream infections in intensive care neonates. Pediatr Infect Dis J. 2007;26:607-612.
2. Baumert N, von Eiff C, Schaaff F, et al. Physiology and antibiotic susceptibility of Staphylococcus aureus small colony variants. Microb Drug Resist. 2002;8:253-260.
3. Klingenberg C, Aarag E, Ronnestad A, et al. Coagulase-negative staphylococcal sepsis in neonates. Association between antibiotic resistance, biofilm formation and the host inflammatory response. Pediatr Infect Dis J. 2005;24:817-822.
4. Krediet TG, Mascini EM, van Rooij E, et al. Molecular epidemiology of coagulase-negative staphylococci causing sepsis in a neonatal intensive care unit over an 11-year period. J Clin Microbiol. 2004;42:992-995.
5. Raimundo O, Heussler H, Bruhn JB, et al. Molecular epidemiology of coagulase-negative staphylococcal bacteraemia in a newborn intensive care unit. J Hosp Infect. 2002;51:33-42.
6. Hira V, Sluijter M, Goessens WH, et al. Coagulase-negative staphylococcal skin carriage among neonatal intensive care unit personnel: from population to infection. J Clin Microbiol. 2010;48:3876-3881.
7. Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285-291.
8. Zhang K, Sparling J, Chow BL, et al. New quadriplex PCR assay for detection of methicillin and mupirocin resistance and simultaneous discrimination of Staphylococcus aureus from coagulase-negative staphylococci. J Clin Microbiol. 2004;42:4947-4955.
9. Standards NCfCL. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically, 4th ed. National Committee for Clinical Laboratory Standards, Wayne, PA. 1997.
10. CLSI. Performance standards for antimicrobial susceptibility testing: 16th informational supplement, vol. 26. Clinical Laboratory Standards Institute, Wayne, PA.; 2006.
11. Vuong C, Kocianova S, Yu J, Kadurugamuwa JL, Otto M. Development of real-time in vivo imaging of device-related Staphylococcus epidermidis infection in mice and influence of animal immune status on susceptibility to infection. J Infect Dis. 2008;198:258-261.
12. van Belkum A, Emonts M, Wertheim H, et al. The role of human innate immune factors in nasal colonization by Staphylococcus aureus. Microbes and infection / Institut Pasteur. 2007;9:1471-1477.
Chapter
5
5.1 Vaccination with SesC decreases Staphylococus
epidermidis biofilm formation
5.2 Inhibition of Staphylococcus epidermidis biofilm formation
by rabbit polyclonal antibodies against the SesC protein
Inhibition of Staphylococcus epidermidis
biofilm formation
66
Chapter
5
5.1 Vaccination with SesC decreases Staphylococus
epidermidis biofilm formation
5.2 Inhibition of Staphylococcus epidermidis biofilm formation
by rabbit polyclonal antibodies against the SesC protein
Inhibition of Staphylococcus epidermidis
biofilm formation
Chapter
5.1
Vishal Hira*Mohammad Shahrooei*
Laleh KhodaprastLadan KhodaprastBenoit StijlemansSoňa Kucharíková
Peter BurghoutPeter W. M. Hermans
Johan van Eldere
* VH and MS contributed equally to this chapter
Infect Immun. 2012;80(10):3660-8
Vaccination with SesC decreases Staphylococus
epidermidis biofilm formation
68
Chapter
5.1
Vishal Hira*Mohammad Shahrooei*
Laleh KhodaprastLadan KhodaprastBenoit StijlemansSoňa Kucharíková
Peter BurghoutPeter W. M. Hermans
Johan van Eldere
* VH and MS contributed equally to this chapter
Infect Immun. 2012;80(10):3660-8
Vaccination with SesC decreases Staphylococus
epidermidis biofilm formation
Chapter 5.170
Abstract
The increased use of medical implants has resulted in a concomitant rise in device-related infections. The majority of these infections are caused by Staphylococcus epidermidis biofilms. Immunoprophylaxis and immunotherapy targeting in vivo-expressed, biofilm-associated, bacterial cell surface-exposed proteins are promising new approaches to prevent and treat biofilm-related infections, respectively. Using an in silico procedure, we identified 64 proteins that are predicted to be S. epidermidis surface exposed (Ses), of which 36 were annotated as (conserved) hypothetical. Of these 36 proteins, 5 proteins -3 LPXTG motif-containing proteins (SesL, SesB, and SesC) and 2 of the largest ABC transporters (SesK and SesM)- were selected for evaluation as vaccine candidates. This choice was based on protein size, number of antigenic determinants, or the established role in S. epidermidis biofilm formation of the protein family to which the candidate protein belongs. Anti-SesC antibodies exhibited the greatest inhibitory effect on S. epidermidis biofilm formation in vitro and on colonization and infection in a mouse jugular vein catheter infection model that includes biofilms and organ infections. Active vaccination with a recombinant truncated SesC inhibited S. epidermidis biofilm formation in a rat model of subcutaneous foreign body infection. Antibodies to SesC were shown to be opsonic by an in vitro opsonophagocytosis assay. We conclude that SesC is a promising target for antibody mediated strategies against S. epidermidis biofilm formation.
Vaccination with SesC decreases S. epidermidis biofilm 71
Ch
ap
ter
5.1
Introduction
Staphylococcus epidermidis is considered to be the major cause of device-related infections, especially catheter-related infections. These infections have increased in number, owing to the increased use of such devices (1). The ability to form biofilms on medical implant surfaces is the main virulence factor of S. epidermidis (2). Biofilms are notoriously resistant to both immune and antimicrobial agents (3, 4). Currently, the only completely effective method for curing biofilm infections is to remove the infected device, which is a risky, costly, and stressful procedure. Different strategies are used against biofilm infections (5). The traditional approach to prevent biofilm formation is administration of bactericidal agents to the patient or the biomaterial (6). Other frequently utilized options involve the modification of biomaterial surface to prevent initiation of bacterial colonization (7-10). However, these strategies have their disadvantages. There is the ineffectiveness of traditional antibacterial compounds due to the nature of biofilms and high prevalence of antimicrobial resistance, there are the induction, generation, and selection of resistance by the slow release of subinhibitory concentrations of antimicrobials from biomaterials, and there are the problems linked to biochemical and chemical compatibility, increased cost, short time effect, effect on mechanical properties, and cytotoxicity (4, 11). Immunoprophylaxis and immunotherapy targeting in vivo expressed biofilm-related proteins and cell surface components are promising new approaches for the prevention and treatment of biofilms. Most vaccines now available for human use are whole (killed or attenuated) microorganisms or subunit vaccines. S. epidermidis is a ubiquitous colonizer of human skin, and prior staphylococcal infections do not cause immunological protection (12). However, this does not imply that immunoprophylaxis and immunotherapy against S. epidermidis biofilms and infections would not be possible. Several recent studies have shown that antibodies against cell surface components of S. epidermidis can affect the rate of biofilm formation or adherence of these bacteria to medical devices in vitro. Cerca et al. (13) showed that antibodies against polysaccharide intercellular adhesion (PIA), which is identical to poly-N-acetylglucosamine (PNAG), readily penetrated the S. epidermidis biofilm and bound to the sessile cells. Sessile bacteria nevertheless exhibited more resistance to opsonic killing than their planktonic counterparts. Using polyclonal antibodies against a fibrinogen-binding protein from S. epidermidis (Fbe), Pei et al. (14) could block adherence of S. epidermidis to fibrogen-coated catheters in vitro. Sun et al. (15) showed that monoclonal antibodies against accumulation-associated protein (Aap) can significantly reduce the accumulation but not initiation phase of S. epidermidis biofilm formation in vitro. Maira-Litran et al. (16) showed that vaccination of rats with purified PIA/PNAG can elicit protective immunity against both CoNS and S. aureus. Hence, surface-expressed components including S. epidermidis surface-exposed “Ses” proteins (17, 18), PIA, and teichoic acids (19, 20) are potential targets for vaccine development.In the present study, we identified potential surface-exposed proteins of S. epidermidis and investigated the potential use of rabbit polyclonal antibodies raised against five “Ses” proteins and against whole (killed) microorganisms for eradication of S. epidermidis biofilms in vitro. For the most immunogenic protein, SesC, we also tested the effect of immunization with recombinant antigen (active immunization) on in vivo biofilm formation and investigated the immunological effector function of specific rabbit polyclonal anti-SesC IgGs (αSesC-IgGs). This was done by challenging animals in a newly developed central venous catheter
Chapter 5.172
murine model with S. epidermidis bacteria preincubated with αSesC-IgGs and by performing an in vitro opsonophagocytosis assay.
Materials & methods
In silico selection of Ses proteins The complete sequence of S. epidermidis ATCC 12228 (21) was retrieved from the National Centre of Biotechnology Information (NCBI) GenBank (http://www.ncbi.nlm.nih.gov/GenBank/). N-terminal signal peptides and transmembrane domains in S. epidermidis proteins were predicted with SignalP and TMHMM (http://www.cbs.dtu.dk/services/). Retention domain prediction lipobox motifs, peptidoglycan-binding domains, choline-binding domains, and LPXTG motives were predicted using the PATTINPROT server (http://npsa-pbil.ibcp.fr/) (22). The prediction of protein subcellular localization was reanalyzed using the online tool PSORTb v.2.0.4 (http://www.psort.org/psortb/). The sequences of all identified Ses proteins were subjected to antigenicity analysis using the “Predicting Antigenic Peptides” server (http://imed.med.ucm.es/Tools/antigenic.pl).
Bacterial strains, plasmids, primers, and mediaFor biofilm inhibition studies, S. epidermidis strain 10b, which is a strong (PIA-dependent) biofilm-forming strain (23) isolated from a patient with a proven catheter-related infection, was used. For recombinant protein production and PCR screening of isolates, the sequences of the selected ses genes were retrieved via the NCBI GenBank from the complete genome of the non-biofilm-forming S. epidermidis strain ATCC 12228. On the basis of these sequences, all primers were designed and purchased from Eurogentec (Seraing, Belgium). Primers used in the present study are listed in Table 1. Each ses gene was PCR-amplified using genomic DNA isolated from strain 10b as a template and sequenced. For recombinant protein production, amplicons were cloned in pET11c (Stratagene, La Jolla, CA). The recombinant plasmids were transformed into Escherichia coli BL21(DE3). S. epidermidis was grown in brain heart infusion broth (BHI; Oxoid) and E. coli was grown in Luria-Bertani medium supplemented with 100 μg of ampicillin/ml when it was transformed with plasmids. Solid medium consisted of the corresponding liquid medium supplemented with 1 to 2% agar.
Bacterial isolates and species identificationA total of 76 S. epidermidis clinical and commensal isolates from hospitalized patients (n = 60), the skin of healthy individuals (n = 11), and five previously described strains (strains 10b and 1457 (23), ATCC 35984/RP62A (18), and ATCC 12228 and TU3298 (24)) were collected. Clinical isolates were recovered from blood cultures of neonates (n = 45) with late-onset sepsis and an intravascular catheter in place at the neonatal intensive care unit of the Erasmus MC–Sophia Children’s Hospital in Rotterdam, Netherlands, and from different clinical specimens from patients (n = 15) hospitalized at University Hospital Gasthuisberg (Katholieke Universiteit Leuven) in Leuven, Belgium. Species identification was performed with Vitek 2 (bioMérieux).
Vaccination with SesC decreases S. epidermidis biofilm 73
Ch
ap
ter
5.1
PCR screening of ses genes in clinical and commensal isolatesWe performed a duplex PCR, amplifying both a ses gene and the 16S rRNA gene. The primers used for PCR screening of ses genes were sesR1 and sesF1 sets, and primers for the 16S rRNA gene were previously described (25). For each strain, genomic DNA was extracted using a QIAamp DNA minikit (Qiagen) with the addition of 30 μg of lysostaphin/ml at the lysis step.
Cloning, expression, and purification of histidine-tagged fusion proteinsRecombinant extracellular domains of the Ses proteins were expressed with hexahistidine tags at their N or C termini using the expression vector pET11c (Stratagene, La Jolla, CA). Each ses gene fragment was PCR amplified using reverse and forward (sesF2 and sesR2 set) primers incorporating flanking NheI and BamHI restriction sites and a sequence coding for a N or C-terminal His6 tag. Amplified products were cloned in NheI- and BamHI-digested pET11c, and the resulting plasmids were transformed into E. coli BL21(DE3). Pure plasmid DNA was isolated using a High Pure plasmid isolation kit (Roche Diagnostics), and PCR amplification and sequencing were used for verification. The recombinant plasmids were used for recombinant protein production as previously described in detail (26).
Table 1. Primers used in this study. Primer Sequence (5’→ 3’)a Useb
sesCF1 GTTGATAACCGTCAACAAGG A
sesCR1 CATGTTGATCTTTTGAATCCC A
sesLF1 TGGGCCACTCAATACAGTCA A
sesLR1 TTGGCGTGTTTCTGTCTTTG A
sesMF1 CAGGTGCCTTGGAATCGC A
sesMR1 GCGTACCTTGCCAGTAGTC A
sesKF1 CCAATTACTAGTATTAAATTCAG A
sesKR1 CTACACTGTTAGACGTGAG A
sesBF1 GCTATGAAAAATAGTGGTGGC A
sesBR1 CGTAGTATGAATTGAGCTCAC A
sesCF2 ACGTGCTAGCGCAGATTCAGAAAGTACATC B
sesCR2 GAACAGCTACAGCTGATCATCACCATCACCATCACTAGGATCCGCAT B
sesLF2 CACGTGCTAGCCATCACCATCACCATCACAAAACGCAAGATGAAGCGAAA B
sesLR2 GGAACTCAAATTATTTATTAAGGATCCGCAT B
sesMF2 ACTGGCTAGCCATCACCATCACCATCACGGGGGCACCTCAAGTACAG B
sesMR2 GTTACACCAGAATCTATCTATTAGGATCCGCAT B
sesKF2 CACGTGCTAGCGCTGAATCAAACACTTCAGTTTCTTCT B
sesKR2 CTATTACCAAATACAGGTATGCATCACCATCACCATCACTAGGATCCGCAT B
sesBF2 ACGTGCTAGCGCAGCCGAAGTAACATCTC B
sesBR2 CTCAATTCATACTACGTAGGTCATCACCATCACCATCACTAGGATCCGCAT BaFlanking restriction sites are underlined and in italics, and sequences coding for an N- or C-terminal HIS6 tag are indicated in boldface. b A, used for PCR screening of ses genes in clinical and commensal isolates; B, used for cloning ses genes in pET11c.
Chapter 5.174
Preparation and purification of polyclonal anti-rSes IgG antibodiesPolyclonal antibodies were produced by Eurogentec by immunization of rabbits with purified rSes proteins according to standard immunization protocols. In addition, ethanol (80%)-killed S. epidermidis ATCC 12228 was used as whole-cell preparation to raise serum against the complete surface of S. epidermidis. Briefly, specific-pathogen-free rabbits were immunized with 100 μg of rSes proteins or killed bacteria, and boosters were given at 14, 28, and 56 days after the first immunization. Preimmune serum was taken before the first immunization. After 87 days, the total blood of the rabbit was collected, and the serum was stored at -20°C.Total IgGs from pre- and postimmune sera were purified by absorption to a protein G column (GE Healthcare) according to the manufacturer’s instructions. Only for SesC were specific rabbit polyclonal IgGs (αSesC-IgGs) further purified using antigen affinity purification, as previously described in detail (26). The purity and reactivity of the purified IgGs against their respective recombinant proteins and ethanol killed cells were determined by Coomassie blue staining of SDS-PAGE electrophoresis gels, enzyme-linked immunosorbent assay (ELISA), and Western blot according to standard protocols. To evaluate the presence of the respective proteins on the surface of S. epidermidis, an indirect ELISA with the pre- and postimmune sera on whole-cell and lysed S. epidermidis ATCC 12228 was performed. To obtain whole-cell S. epidermidis, the sediment of an overnight culture was resuspended in 1 ml of 0.9% NaCl and heated for 30 min at 56°C. For lysed cells, the sediment of an overnight culture was resuspended in 1 ml of 0.9% NaCl with 100 μg of lysostaphin (Sigma-Aldrich)/ml. Subsequently, the suspension was incubated at 37°C for 4 h in rotating tubes. For coating ELISA plates, the suspensions were diluted to 107 CFU/ml in 0.9% NaCl. Hereafter, 100 μl of the dilution was applied to each well on the plate, effectively coating each well with 106 CFU. Coated plates were incubated overnight at 4°C before the test was performed according to standard protocols (27). The test was considered positive when the optical density at 405 nm (OD405) of postimmune sera was at least twice as high as the OD405 of preimmune sera after subtraction of the average OD405 of the blank wells. In vitro biofilm inhibition assays Using a semiquantitative microtiter plate method (15, 28), the effect of pre- and postimmune IgGs against rSes proteins on in vitro biofilm formation during the first 2 h (primary attachment) and overnight (accumulation and establishment phase) was studied. For quantification of biofilms, the following procedure was used. A 20-μl aliquot of frozen cultures of S. epidermidis strains 10b was inoculated into 5 ml of BHI and grown to the late-exponential/stationary-growth phase in a shaking incubator at 37°C. Cultures were subsequently diluted in BHI to an OD600 of 0.005 (5 × 106 CFU/ml) in fresh BHI. To evaluate the effect of IgGs on the primary attachment of S. epidermidis strain 10b, these starting cultures with an OD600 of 0.005 were grown at 37°C to an OD600 of 1. Cultures were subsequently mixed with either pre- or postimmune IgGs (10 μg/ml) and, after 2 h of incubation at 4°C, 200-μl portions of the mixtures were pipetted into 96-well polystyrene microtiter plates (BD Biosciences, Heidelberg, Germany), followed by incubation for 2 h at 37°C without shaking. To study the effect of IgGs on overnight biofilm formation, the cultures diluted to an OD600 of 0.005 were mixed with either pre- or postimmune IgGs (10 μg/ml) and, after 2 h of incubation at 4°C, 200 μl of the mixtures (106 cell per well) was added to each well of 96-well polystyrene microtiter plates, followed by incubation overnight at 37°C without shaking. After the incubation, the plates were washed three times with phosphate-buffered saline
Vaccination with SesC decreases S. epidermidis biofilm 75
Ch
ap
ter
5.1
(PBS [pH 6.8], containing 0.5 M NaCl and 10 mM EDTA), and adherent biofilms were stained with 200 μl of 1% (wt/vol) crystal violet (Sigma) for 10 min, after which the plates were washed three times with water and dried. For quantification, 160 μl of 30% (vol/vol) acetic acid was added to each well to dissolve the stain. The OD595 of the dissolved stain was measured in a multipurpose UV/VIS plate reader. The percent inhibition of biofilm formation was calculated by using the following formula: (A595, positive − A595, antibody)/(A595, positive − A595, negative) × 100 (15, 26). The average inhibition for each pre- or postimmune IgG was obtained from at least eight independent measurements generated in at least two independent experiments. S. epidermidis strain 10b in BHI without any added IgG was used as positive control, and BHI without bacteria was used as a negative control. Biofilm formation in immunized rats For the vaccination experiments, ex-germ-free Fisher (EGF) rats (n = 6 per experiment) were divided into two groups of three rats each. Each rat in the first group was immunized twice intraperitoneally with first 100 μg of rSesC (dissolved in normal saline) in complete Freund adjuvant (CFA) and 2 weeks later a second time with 50 μg of rSesC (dissolved in normal saline) in incomplete Freund adjuvant (IFA). The second group of rats was injected with the same volume of normal saline in CFA and IFA but without antigen. Two weeks after the second immunization, the immune response of pre- and postimmunization sera of all rats was tested by ELISA. Subsequently, five catheter (Arrow International, Reading, PA) fragments preincubated with S. epidermidis 10b bacteria (±104 cells/catheter) were implanted in each rat and 24 h later explanted as previously described (26), and the numbers of sessile cells were quantified by CFU counting as previously described (29). Briefly, after gentle cleaning with 0.9% NaCl, the catheters were placed in a tube containing 1 ml of 0.9% NaCl. Tubes were vortex mixed for 10 s, sonicated for 10 min at 40 kHz in a water bath (Branson 2200; Branson Ultrasonics), and vortex mixed again for 10 s. Thereafter, tube contents were diluted and 50-μl aliquots of 10-fold dilutions were plated on tryptone soy agar (TSA; Oxoid) plates, using a spiral plating system, and the plates were incubated at 37°C overnight. Colonies on all plates were counted, and the number of bacteria was defined as the mean of at least five quantitative cultures. All animal experiments were repeated at least twice and conducted in compliance with the guidelines for animal experimentation. All experimental protocols were approved by the Institutional Animal Care Commission and Ethical Committee of the KU Leuven. Biofilm formation in jugular vein-catheterized (JVC) mouse model In order to investigate the effector function of αSesC-IgGs, we developed and used a previously described central venous catheter murine model (30, 31) that reflects the clinical situation of catheter colonization by contaminated infusions. Briefly, 4-weeks-old Swiss-Webster mice from Taconic (n = 9 mice per experiment, with the experiment repeated twice) were divided into three groups of three mice each. Mice were anesthetized with sodium pentobarbital (nembutal, 40 to 60 mg/kg [body wt]) injected intraperitoneally. The animal was then placed in dorsal recumbency under a dissecting microscope. A small vertical incision was made using a scalpel, and the right jugular vein was identified, mobilized, and exposed with blunt surgical dissection. A single lumen polyethylene catheter (internal diameter, 28 mm; outer diameter, 61 mm; length, 6 cm; insertion length, 1 cm; Intramedic [Becton Dickinson, catalog no. 427400]) was inserted into the right jugular vein and advanced into the superior vena cava via a small incision in the vein made with vein
Chapter 5.176
scissors. A ligature was then tied loosely around the catheter, and patency was verified. Once blood flow had been established, the catheter was anchored in place and flushed with 10 μl of saline. Subsequently, a small midline skin incision was made between the scapulae. The catheter port was tunneled back through the scapular incision. The incisions were then closed with stitches. Mice were housed separately and monitored for recovery. An overnight culture of S. epidermidis strain 10b, grown to the late exponential/stationary growth phase in BHI, was pelleted, resuspended, and diluted to an OD600 of 0.3 (±3E+08) in 0.9% NaCl. Three inoculums were taken, one without any IgG, while two others were mixed with either preimmune or αSesC-IgGs (80 μg/ml) and incubated for 2 h at 4°C. After a 24-h recovery from surgery, 150 μl (±5E+07) of the mixture was injected through the catheter lumen. Animals in the first, second, and third groups received bacteria, bacteria preincubated with preimmune IgGs, and bacteria preincubated with αSesC-IgGs, respectively. After the blood samples were obtained at day 5 postinfection, the animals were sacrificed. The catheters were aseptically removed, and the portion (1 cm) that was inside the vein was cut, gently washed, and processed for quantitative culturing as explained above. To compare the overall infection rate, the spleens, livers, hearts, veins, and right kidneys were aseptically harvested, mechanically homogenized in 0.9% NaCl, and processed for quantitative cultures as explained above. Opsonophagocytosis of planktonic and biofilm bacteria by αSesC-IgGs To prepare the bacteria for the evaluation of susceptibility to opsonic killing, the overnight culture of S. epidermidis 10b in BHI was pelleted for 5 min at 12,000 × g at 4°C, washed twice with PBS, and subsequently diluted to an OD600 of 1. For evaluation of opsonophagocytosis of planktonic cells, 10 μl of bacterial suspension was used, and for evaluation of opsonophagocytosis of biofilm cells, 5-mm catheter (Arrow International) fragments were added to the bacterial suspension, incubated at 37°C for 2 h, and subsequently washed with 1 ml of PBS. The opsonophagocytosis assay was performed with fresh blood obtained from human healthy volunteers as previously described (32), with some modifications. Briefly, fresh whole blood from two volunteers was collected in vacuum blood collection tubes containing the anticoagulant heparin and then aliquoted into 1.5-ml microcentrifuge tubes (0.5 ml/tube). Catheter fragments preincubated with S. epidermidis strain 10b or planktonic bacteria (10 μl of bacterial suspension with an OD600 of 1) after 1 h preincubation at 4°C with preimmune or αSesC-IgGs (30 μg/ml) or the same volume of PBS or nothing were added to the 1.5-ml microcentrifuge tubes containing 0.5 ml of fresh blood. Tubes were incubated at room temperature with gentle rocking and, after 30 min, the samples containing planktonic bacteria were serially diluted and plated onto TSA plates to determine the number of surviving CFU. For samples containing catheter fragments, catheter fragments were first removed and gently washed with 1 ml of saline and then processed for quantification of the number of surviving cells on the catheter fragments as explained above. In order to determine the input CFU in samples containing planktonic or sessile bacteria attached to the catheter fragments, a portion the of untreated planktonic samples was serially diluted and plated onto TSA plates, or the number of bacteria attached to the untreated catheter fragments was quantified as explained above. All samples were assayed in triplicate, and all experiments were repeated at least twice.
Vaccination with SesC decreases S. epidermidis biofilm 77
Ch
ap
ter
5.1
Statistical analysis In the present study, for all of the in vitro and in vivo experiments, data were pooled from two independent experiments. For all data from the bacterial adherence and opsonophagocytosis assays, two hypotheses were tested. A significant change in the adherence levels or survival rates for different IgGs (preimmune or anti-Ses IgGs) was tested with a one-way analysis of variance (ANOVA). A significant difference in adherence for an experiment set including pre- and postimmune IgGs from a particular rabbit was tested with a two-way ANOVA. When the one-way ANOVA was significant, two-sided univariate tests with a correction for multiple comparisons were performed (Bonferroni test) to locate the significant differences. For in vivo experiments, differences between the control and vaccine groups or between groups injected with bacteria treated with preimmune or αSesC-IgGs were examined between the number of bacteria recovered from the catheter fragments (in both in vivo models) or from the organs (only JVC model) using one-way ANOVA. A P value of <0.05 was considered significant. Results Selection of Ses proteins In order to identify the potential Ses proteins we used our in silico selection procedure summarized in the diagram shown in Figure 1. In total, from the 2,419 predicted S. epidermidis proteins, 64 proteins were identified as Ses proteins. Of these, 36 were (conserved) proteins with a hypothetical function (see Supplemental Table). LPXTG motif-containing and ABC transporter lipoproteins are two major types of cell surface proteins that may play important roles in the pathogenesis of S. epidermidis infections (33, 34). We selected three predicted LPXTG motif-containing proteins and two predicted lipobox-containing ABC transporter proteins of which the function had not yet been characterized. These were based on the protein size, the number of antigenic determinants and the importance of the protein family, to which the candidate protein belongs, in S. epidermidis biofilm formation and pathogenesis (Table 2).
S. epidermidis proteins
+ SP
- SP
2056 Intracellular proteins
363 proteins
174 proteins<2 TM
2 TM
>2 TM
31 proteins
158 Transmembrane proteins
49 Lipobox
6 PBD
9 LPxTG
0 CBD
+Lipbox/PBD/CBD/LPxTG
-Lipbox/PBD/ CBD/LPxTG
+LPxTG
-LPxTG
141 Excreted proteins
2 proteins
62 proteins
Selected for Expression
2
3
Figure 1. In silico selection of genes coding for “surface-exposed proteins” of S. epidermidis. SP, signal peptide; TM, transmembrane helix; PBD, peptidoglycanbinding domain; CBD, choline-binding domains.
Chapter 5.178
Presence of ses genes in S. epidermidis isolates We hypothesized that the ideal targets for immunoprophylaxis or immunotherapy against S. epidermidis biofilms would be surface components that were conserved across the species, in particular those which are highly expressed in the bloodstream and in biofilms, with a possible role in biofilm formation or an essential function. To verify the conserved nature of the selected genes, we investigated the distribution of ses genes in clinical and commensal isolates of S. epidermidis. Gene-specific PCR amplification of sesM, sesB, and sesC was positive in all tested S. epidermidis isolates (n = 76); for sesL this was 68% (n = 52 of 76 isolates), and for sesK this was 9% (n = 7 of 76 isolates). There was no significant difference in the prevalence of the sesL and sesK genes in clinical or commensal isolates. We also studied the in vitro and in vivo expression of five selected ses genes in planktonic and sessile bacteria, as previously explained (26). Gene expression studies showed that the selected ses genes are expressed at the level of the transcriptome in both planktonic and sessile bacteria in vitro and in vivo. However, the in vitro and in vivo expression patterns of ses genes in planktonic and biofilm cells varied widely (data not shown). Recognition of Ses proteins by polyclonal antibodies The purity and reactivity of the purified IgGs against their respective recombinant proteins and ethanol-killed cells were determined by Coomassie blue staining of SDS-PAGE gels, ELISA, and Western blotting according to standard protocols. Figure 2 shows examples of such evaluations. To evaluate the recognition of selected Ses proteins by the antisera and their expression on the surface, we performed Western blots and ELISAs on purified recombinant proteins and S. epidermidis ATCC 12228 lysate for each anti-Ses serum (Table 3). For antiserum against SesC, SesL, and SesB, both Western blotting and ELISA were positive against the respective recombinant protein and bacterial lysate. ELISA against whole-cell S. epidermidis ATCC 12228 was also positive for these antisera, as was whole-cell antiserum against these three recombinant proteins. For antiserum against SesM, Western blotting and ELISA against SesM recombinant protein and S. epidermidis lysate were positive, but ELISA against whole-cell S. epidermidis was negative. The latter was also true for antiserum against SesK, but additionally, Western blot results against whole-cell S. epidermidis for this antiserum and whole-cell antiserum against rSesK were negative as well. These data suggest that the selected proteins are surface exposed except possibly for SesK and SesM, which are not expressed under the chosen culture conditions for S. epidermidis.
Table 2. Ses proteins selected by in silico procedure and used in this study. Locus Putative product Protein
accession no.Protein size (no. of
amino acids)Motif No. of antigenic
determinants
SE2232 conserved hypothetical protein (SesC) NP_765787.1 676 LPXTG 20
SE1106 ABC transporter _membrane spanning protein (SesL) NP_764661.1 564 Lipobox 16
SE1981 nickel ABC transporter/ nickel-binding protein (SesM) NP_765536.1 491 Lipobox 18
SE1501 hypothetical protein (SesK) NP_765056.1 415 LPXTG 11
SE2152 hypothetical protein (SesB) NP_765707.1 196 LPXTG 7
Vaccination with SesC decreases S. epidermidis biofilm 79
Ch
ap
ter
5.1
Biofilm inhibition by purified polyclonal anti-rSes-IgGs in vitro Of the five antibodies, the maximum inhibition of initial attachment and overnight biofilm formation of the bacteria on polystyrene surfaces (Figure 3A and B), observed in these in vitro experiments, occurred when bacteria were preincubated with IgGs purified from the serum of the rabbit immunized with rSesC. This reduction for overnight biofilm formation was however not statistically significant (P < 0.01 and P > 0.05, one- and two-way ANOVAs). Effect of immunization with rSesC on biofilm formation In order to test the potential of SesC as a target for vaccination against S. epidermidis biofilms, rats were actively immunized with rSesC. The number of sessile bacteria attached to the catheter fragments 24 h after implantation in the group immunized with rSesC compared to the normal saline-immunized group was significantly reduced (20-fold; P < 0.01; one-way ANOVA) (Figure 4).
Figure 2. (A) Coomassie-stained SDS-PAGE gel (12%) of rSes. Lane 1, SDS-PAGE marker; lane 2, total E. coli extract after induction of SesC expression and cell lysis; lane 3, protein extract after binding the His-tagged SesC to the nickel column; lane 4, protein extract after washing the nickel column; lane 5, SesC recombinant protein after elution; lane 6, negative control. (B) Affinity of preimmune (empty squares) and aSesC-IgGs (solid squares) to rSesC. An indirect ELISA was performed using a 96-well ELISA plate coated with rSesC. IgGs were added to each well and incubated for 3 h at 37°C. Bound IgGs were measured at OD405 with an alkaline phosphatase-conjugated anti-rabbit immunoglobulin. x and y axes indicate the log10 IgG concentration (ng/ml) and the OD405 absorbance, respectively.
A B
Table 3. Western blot and ELISA data obtained using immune sera against recombinant Ses proteins and whole-cell S. epidermidis ATCC 12228a
On respective rSes protein On S. epidermidis lysate on WC S. epidermidisAntiserum target WB ELISA WB ELISA ELISASesC + + + + +SesL + + + + +SesM + + + + -SesK + + - + -SesB + + + + +WC +* + + + +a WB, Western blot; WC, Whole cell. *, Western blot with whole-cell antiserum on recombinant proteins was positive for all proteins except SesK.
Chapter 5.180
Effect of αSesC-IgGs on the overall infection rate in the JVC mouse model To investigate the effector function of αSesC-IgG antibodies in vivo, the mean numbers of bacteria recovered from the blood, catheters, veins, livers, spleens, hearts, and kidneys of animals in different groups were compared. Preincubation of bacteria with αSesC-IgGs could significantly reduce the number of bacteria recovered from the catheter, vein, spleen, heart, liver, and kidney by 26-, 71.5-, 331-, 327-, 215-, and 52-fold, respectively (P < 0.01, one-way ANOVA), whereas preincubation of bacteria with preimmune IgGs had no effect on the infection rates (Figure 5). Almost no bacteria were found in the circulation.
Figure 3. Effect of total IgGs purified from preimmune (white bars) and anti-Ses (L, K, M, B, and C) and anti-whole-cell (WC) sera (black bars) on primary attachment (A) and overnight biofilm formation (B) of S. epidermidis strain 10b on a polystyrene surface. Overnight cultures were diluted, mixed with IgGs, incubated for 2 h at 4°C, and then transferred into the wells. After 2 h (A) or 14 h (B) of incubation at 37°C, the plates were washed and stained with crystal violet, and the OD595 was measured. The error bars indicate the standard deviations. The data are the averages of eight measurements in two independent experiments. Biofilm inhibition was as defined in the text.
Figure 4. Effect of immunization of EGF rats with rSesC on S. epidermidis 10b biofilm formation. Rats (n = 6) were divided into two groups of three rats each. Rats in the vaccine group were immunized twice intraperitoneally with rSesC. Rats in the control group were injected with the same volume of normal saline. At 2 weeks after the second immunization, the immune response was tested by ELISA. Subsequently, catheter fragments preincubated with S. epidermidis strain 10b (±104 cells/catheter) were implanted in rats and 24 h later explanted, and the numbers of adherent bacteria on the catheters were quantified. The experiment was repeated twice. Data for each group were obtained from adherent bacteria to 30 catheter fragments implanted in six rats (five catheters per animal, three rats per group in each experiment, two groups, two independent experiments). The error bars indicate the standard errors of the mean.
Vaccination with SesC decreases S. epidermidis biofilm 81
Ch
ap
ter
5.1
αSesC-IgGs are opsonic and mediate opsonophagocytosis of planktonic and biofilm cellsIncubation of bacteria in the planktonic or sessile state with whole blood for 30 min led to a significant reduction (>1 log10) in PBS-treated versus input bacteria (P < 0.001) (Figure 6). Preincubation of bacteria in both planktonic and sessile forms with αSesC-IgGs significantly enhanced the opsonophagocytic killing of bacteria (P < 0.001, one-way ANOVA) compared to bacteria treated with preimmune IgGs or PBS (Figure 6). There was no difference between bacteria treated with preimmune IgGs and PBS. αSesC-IgGs showed the same level of enhancement of opsonophagocytic killing of bacteria for both planktonic and sessile bacteria.
Figure 5. Effect of preincubation of S. epidermidis strain 10b with preimmune or αSesC-IgGs on the overall infection rates in the JVC mouse model. A single lumen polyethylene catheter was surgically inserted into the right jugular vein of Swiss-Webster mice. After 24 h, an overnight culture of S. epidermidis strain 10b (±3E+08) in 0.9% NaCl was mixed with preimmune or αSesC-IgGs (80 μg/ml) or the same volume of PBS, followed by incubation for 2h at 4°C. After a 24-h recovery from surgery, 150 μl (±5E+07) of the mixture was injected through the catheter lumen. After 5 days, the overall infection rates in different organs and the catheter colonization were quantified. Data for each group were obtained from bacteria recovered from the catheter or organs of six infected mice (three mice per group in each experiment, three groups, two independent experiments). The error bars indicate the standard errors of the mean.
Figure 6. Effect of preincubation of S. epidermidis strain 10b with αSesC-IgGs on phagocytosis of planktonic and sessile bacteria by human neutrophils. Bacteria grown in planktonic form or attached to catheter fragments were tested for their ability to survive in human blood after preincubation with preimmune IgGs or αSesC-IgGs. Surviving bacteria were measured by viable counting. The data are presented as the mean log10 of surviving CFU. The data for each group are the average of six measurements in two independent experiments. The error bars represent the standard deviation of the measurements.
Chapter 5.182
Discussion
In this study we tried to identify new potential target(s) for immunoprophylaxis and immunotherapy against S. epidermidis biofilm formation. Using our in silico selection method, we first identified potential Ses proteins. Nine of these proteins were proteins with an LPXTG motif (17, 18). Five LPXTG motif-containing proteins (Aap, Bhp, SdrF, SdrG, and SesI) are known to play important roles in the pathogenesis of S. epidermidis infections (35-39). In publicly available genomes of S. epidermidis strains RP62A and ATCC 12228, respectively, 11 and 10 genes encoding LPXTG motif-containing proteins have been identified (17), including those already mentioned. Except for the five LPXTG motif-containing proteins mentioned above, the role of other LPXTG motif-containing proteins has not been studied yet. The second major type of cell surface proteins in Gram-positive bacteria are lipoproteins that participate in a wide range of cell envelope functions (40). The major functional category of lipoproteins are the solute-binding proteins of ABC transport systems for the import of a diverse range of substrates (41). Due to the importance of these two families of proteins, five proteins with unknown function, i.e., three hypothetical LPXTG motif-containing proteins (SesC, SesK, and SesB) and two ABC transporters (SesL and SesM), were selected for further studies. This selection was also based on the protein size and the number of antigenic determinants. We showed by Western blotting that the recombinant proteins of the selected proteins except for SesK were recognized by whole-cell antisera, suggesting these proteins, except possibly for SesK, are most likely surface exposed. Anti-SesK and anti-SesM antisera did not recognize whole cells, suggesting that SesK and SesM are not (sufficiently) expressed under the culture conditions chosen. In addition, sesK is in only present in <10% of tested isolates. Total IgGs isolated from the hyperimmune sera of rabbits immunized with rSesC showed the highest inhibition effect on primary attachment and biofilm formation in vitro, indicating a possible role for this protein in S. epidermidis biofilm formation. The lower effect of IgGs isolated from the sera of animals immunized with other Ses proteins or whole cells can be due to either a low expression level of selected Ses proteins on the surface during biofilm formation or to the fact that the concentration of antibodies used was too low. Also, some of the selected proteins may not be accessible to antibodies in the biofilm structure.Based on the in vitro biofilm inhibition studies, SesC seemed to be the most promising target for prevention and treatment of S. epidermidis biofilms. We therefore selected SesC for further studies. Unfortunately, attempts to create sesC knockout mutants have been unsuccessful, and natural S. epidermidis sesC mutants have not been found yet. However, there are indications that SesC might be a potential fibrinogen-binding protein, playing a role in the attachment to abiotic surfaces (26). Further studies on the function of SesC in biofilm formation are therefore warranted. Our in vivo rat model, although closely resembling the subcutaneous models for catheter-related infections and mimicking intraoperative contamination with skin flora, does not mimic conditions found in the human intravascular system. Intravascular devices are nevertheless the most frequently used medical devices. In addition, the immune response at the site of infection in our subcutaneous rat model may not reflect the immune response to the intravascular device-related infections in peripheral blood. Hence, we developed and used our JVC model to investigate the immune response and effector function of antibodies. The primary functions of antibodies are neutralization and opsonization. The effect of αSesC-IgGs on S. epidermidis biofilms in vitro, in the absence of immune system components,
Vaccination with SesC decreases S. epidermidis biofilm 83
Ch
ap
ter
5.1
suggested a neutralizing effect of αSesC-IgGs or blocking of the function of SesC. However, in vivo, in addition to neutralization, antibodies can opsonize their ligand, thus facilitating its uptake and destruction by natural killer cells, activating complement, and enhancing phagocytosis. The effect of αSesC-IgG antibodies on opsonophagocytosis of planktonic cells in vitro indicates their potential opsonic activity, whereas the reduction of the number of sessile bacteria on catheters in this experiment can be due to both neutralization and the opsonic activity of αSesC-IgG. It is possible that binding by αSesC-IgG of SesC on the surface of sessile bacteria triggers their detachment from catheter fragments and subsequent phagocytosis by neutrophils. The 20-fold reduction of attached bacteria in the vaccinated group compared to the control group suggests a significant expression of SesC in sessile bacteria and is consistent with a role of this protein in S. epidermidis biofilm formation. This is also in line with our in vitro data and a previous report that subcutaneous injection of anti-SesC-IgGs at the place of implantation of catheter fragments reduced the number of attached bacteria to the catheter fragments 60-fold compared to the control group treated with preimmune IgGs (26). The JVC mouse model is a more physiological model for investigating the mechanism of action of antibodies on S. epidermidis biofilms and organ infections, since the reduction in the overall infection rate in JVC murine model can be due to both neutralization and the opsonization activity of αSesC-IgG antibodies. The clear effect of the αSesC-IgG antibodies on the organ infections suggest, on the one hand, a significant role of sesC in tissue infections and, on the other hand, confirm the hypothesis that αSesC-IgG antibodies work in preventing the adherence of the bacteria. In conclusion, antibodies against recombinant SesC were shown to be both neutralizing and opsonic to S. epidermidis and can inhibit biofilm formation in vitro and in vivo. Active vaccination with SesC and preincubation of bacteria with αSesC-IgGs showed a reduction of biofilm formation and infection rates in vivo. The findings of the present study are consistent with those of Shahrooei et al. (26), who reported that SesC might play an important role in S. epidermidis biofilms and be a promising target for immunoprophylaxis and immunotherapy of S. epidermidis biofilms. The precise role of SesC in S. epidermidis biofilm formation remains to be identified. Acknowledgements The authors wish to thank Marcel Sluijter and Theo Hoogenboezem for their excellent technical assistance.
Chapter 5.184
References
1. Otto M. Staphylococcus epidermidis--the ‘accidental’ pathogen. Nat Rev Microbiol. 2009;7:555-567.2. Raad I, Alrahwan A, Rolston K. Staphylococcus epidermidis: emerging resistance and need for alternative
agents. Clin Infect Dis. 1998;26:1182-1187.3. Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science.
1999;284:1318-1322.4. Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135-138.5. McCann MT, Gilmore BF, Gorman SP. Staphylococcus epidermidis device-related infections: pathogenesis and
clinical management. J Pharm Pharmacol. 2008;60:1551-1571.6. Danese PN. Antibiofilm approaches: prevention of catheter colonization. Chem Biol. 2002;9:873-880.7. Illingworth B, Bianco RW, Weisberg S. In vivo efficacy of silver-coated fabric against Staphylococcus
epidermidis. J Heart Valve Dis. 2000;9:135-141.8. Kockro RA, Hampl JA, Jansen B, et al. Use of scanning electron microscopy to investigate the prophylactic
efficacy of rifampin-impregnated CSF shunt catheters. J Med Microbiol. 2000;49:441-450.9. van der Borden AJ, van der Werf H, van der Mei HC, Busscher HJ. Electric current-induced detachment of
Staphylococcus epidermidis biofilms from surgical stainless steel. Appl Environ Microbiol. 2004;70:6871-6874.10. Yu B, Davis EM, Hodges RS, Irvin RT, Li DY. Surface nanocrystallization of stainless steel for reduced biofilm
adherence. Nanotechnology. 2008;19:335101.11. Yu B, Lesiuk A, Davis E, Irvin RT, Li DY. Surface nanocrystallization for bacterial control. Langmuir.
2010;26:10930-10934.12. Van Mellaert L, Shahrooei M, Hofmans D, Eldere JV. Immunoprophylaxis and immunotherapy of Staphylococcus
epidermidis infections: challenges and prospects. Expert review of vaccines. 2012;11:319-334.13. Cerca N, Jefferson KK, Oliveira R, Pier GB, Azeredo J. Comparative antibody-mediated phagocytosis of
Staphylococcus epidermidis cells grown in a biofilm or in the planktonic state. Infect Immun. 2006;74:4849-4855.
14. Pei L, Flock JI. Functional study of antibodies against a fibrogenin-binding protein in Staphylococcus epidermidis adherence to polyethylene catheters. J Infect Dis. 2001;184:52-55.
15. Sun D, Accavitti MA, Bryers JD. Inhibition of biofilm formation by monoclonal antibodies against Staphylococcus epidermidis RP62A accumulation-associated protein. Clin Diagn Lab Immunol. 2005;12:93-100.
16. Maira-Litran T, Kropec A, Goldmann D, Pier GB. Biologic properties and vaccine potential of the staphylococcal poly-N-acetyl glucosamine surface polysaccharide. Vaccine. 2004;22:872-879.
17. Bowden MG, Chen W, Singvall J, et al. Identification and preliminary characterization of cell-wall-anchored proteins of Staphylococcus epidermidis. Microbiology. 2005;151:1453-1464.
18. Gill SR, Fouts DE, Archer GL, et al. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J Bacteriol. 2005;187:2426-2438.
19. Gotz F. Staphylococci in colonization and disease: prospective targets for drugs and vaccines. Curr Opin Microbiol. 2004;7:477-487.
20. Sellman BR, Howell AP, Kelly-Boyd C, Baker SM. Identification of immunogenic and serum binding proteins of Staphylococcus epidermidis. Infect Immun. 2005;73:6591-6600.
21. Zhang YQ, Ren SX, Li HL, et al. Genome-based analysis of virulence genes in a non-biofilm-forming Staphylococcus epidermidis strain (ATCC 12228). Mol Microbiol. 2003;49:1577-1593.
22. Wegmann U, O’Connell-Motherway M, Zomer A, et al. Complete genome sequence of the prototype lactic acid bacterium Lactococcus lactis subsp. cremoris MG1363. J Bacteriol. 2007;189:3256-3270.
23. Van Wijngaerden E, Peetermans WE, Vandersmissen J, et al. Foreign body infection: a new rat model for prophylaxis and treatment. J Antimicrob Chemother. 1999;44:669-674.
24. Mack D, Siemssen N, Laufs R. Parallel induction by glucose of adherence and a polysaccharide antigen specific for plastic-adherent Staphylococcus epidermidis: evidence for functional relation to intercellular adhesion. Infect Immun. 1992;60:2048-2057.
25. Allgaier H, Jung, G., Werner, R.G., Schneider, U., Zahner, H. Elucidation of the Structure of Epidermin, a Ribosomally Synthesized, Tetracyclic Heterodetic Polypeptide Antibiotic. Angewandte Chemie International Edition in English. 1985;24:1051-1053.
26. Vandecasteele SJ, Peetermans WE, Merckx R, Van Eldere J. Quantification of expression of Staphylococcus epidermidis housekeeping genes with Taqman quantitative PCR during in vitro growth and under different conditions. J Bacteriol. 2001;183:7094-7101.
27. Shahrooei M, Hira V, Stijlemans B, et al. Inhibition of Staphylococcus epidermidis biofilm formation by rabbit polyclonal antibodies against the SesC protein. Infect Immun. 2009;77:3670-3678.
28. Bogaert D, Veenhoven RH, Ramdin R, et al. Pneumococcal conjugate vaccination does not induce a persisting mucosal IgA response in children with recurrent acute otitis media. Vaccine. 2005;23:2607-2613.
Vaccination with SesC decreases S. epidermidis biofilm 85
Ch
ap
ter
5.1
29. Christensen GD, Simpson WA, Younger JJ, et al. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985;22:996-1006.
30. Pintens V, Massonet C, Merckx R, et al. The role of sigmaB in persistence of Staphylococcus epidermidis foreign body infection. Microbiology. 2008;154:2827-2836.
31. Lazzell AL, Chaturvedi AK, Pierce CG, et al. Treatment and prevention of Candida albicans biofilms with caspofungin in a novel central venous catheter murine model of candidiasis. J Antimicrob Chemother. 2009;64:567-570.
32. Oosterlinck W, Vanderper A, Flameng W, Herijgers P. Glucose tolerance and left ventricular pressure-volume relationships in frequently used mouse strains. Journal of biomedicine & biotechnology. 2011;2011:281312.
33. Smith EJ, Visai L, Kerrigan SW, Speziale P, Foster TJ. The Sbi protein is a multifunctional immune evasion factor of Staphylococcus aureus. Infect Immun. 2011;79:3801-3809.
34. Desvaux M, Dumas E, Chafsey I, Hebraud M. Protein cell surface display in Gram-positive bacteria: from single protein to macromolecular protein structure. FEMS Microbiol Lett. 2006;256:1-15.
35. Scott JR, Barnett TC. Surface proteins of gram-positive bacteria and how they get there. Annual review of microbiology. 2006;60:397-423.
36. Arrecubieta C, Lee MH, Macey A, Foster TJ, Lowy FD. SdrF, a Staphylococcus epidermidis surface protein, binds type I collagen. J Biol Chem. 2007;282:18767-18776.
37. Cucarella C, Solano C, Valle J, et al. Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J Bacteriol. 2001;183:2888-2896.
38. Davis SL, Gurusiddappa S, McCrea KW, Perkins S, Hook M. SdrG, a fibrinogen-binding bacterial adhesin of the microbial surface components recognizing adhesive matrix molecules subfamily from Staphylococcus epidermidis, targets the thrombin cleavage site in the Bbeta chain. J Biol Chem. 2001;276:27799-27805.
39. Hussain M, Herrmann M, von Eiff C, Perdreau-Remington F, Peters G. A 140-kilodalton extracellular protein is essential for the accumulation of Staphylococcus epidermidis strains on surfaces. Infect Immun. 1997;65:519-524.
40. Soderquist B. Surgical site infections in cardiac surgery: microbiology. APMIS. 2007;115:1008-1011.41. Sutcliffe IC, Harrington DJ. Pattern searches for the identification of putative lipoprotein genes in Gram-
positive bacterial genomes. Microbiology. 2002;148:2065-2077.42. Sutcliffe IC, Harrington DJ. Putative lipoproteins of Streptococcus agalactiae identified by bioinformatic
genome analysis. Antonie van Leeuwenhoek. 2004;85:305-315.
Chapter 5.186
Supplemental Table List of surface-exposed proteins as predicted by in silico analysis.
Locus Putative product Protein
size (no. of amino acids)
MotifNo. of
antigenic determinants
SE2395 Ser-Asp rich fibrinogen-binding,bone sialoprotein-binding protein 1633 LPXTG 50
SE0175 accumulation-associated protein 1469 LPXTG 35
SE0331 Ser-Asp rich fibrinogen-binding, bone sialoprotein-binding protein 1056 LPXTG 29
SE1500 hypothetical protein 1012 LPXTG 40
SE0828 lipoprotein VsaC 827 LPXTG 31
SE1238 penicillin-binding protein 3 696 PBD 21
SE2232 conserved hypothetical protein 676 LPXTG 20
SE1106 ABC transporter _membrane spanning protein 564 Lipobox 16
SE0684 peptide binding protein OppA 547 Lipobox 19
SE1981 nickel ABC transporter nickel-binding protein 491 Lipobox 18
SE1511 protoporphyrinogen oxidase 482 PBD 22
SE1501 hypothetical protein 415 LPXTG 11
SE1588 conserved hypothetical protein 402 Lipobox 13
SE2212 conserved hypothetical protein 388 Lipobox 16
SE0800 potD protein 357 Lipobox 12
SE1628 hypothetical protein 357 LPXTG 8
SE0424 lipase LipA 349 PBD 12
SE0518 ferrichrome ABC transporter 347 Lipobox 12
SE1768 ferrichrome ABC transporter 334 Lipobox 10
SE0193 Zn-binding lipoprotein adcA 331 Lipobox 11
SE1521 prsA 325 Lipobox 10
SE1070 thioredoxine reductase 325 Lipobox 9
SE0167 periplasmic-iron-binding protein BitC 324 Lipobox 14
SE2319 autolysin _N-acetylmuramoyl-L-alanine amidase 324 PBD 10
SE0224 glycine betaine_carnitine_choline ABC transporter _osmoprotec_ opuCC 319 Lipobox 12
SE0391 conserved hypothetical protein 317 Lipobox 12
SE0405 lipoprotein 309 Lipobox 10
SE2157 conserved hypothetical protein 307 Lipobox 13
SE2222 conserved hypothetical protein 304 Lipobox 11
SE2223 conserved hypothetical protein 300 Lipobox 11
SE0149 hypothetical protein 300 Lipobox 9
SE0522 conserved hypothetical protein 296 Lipobox 8
Vaccination with SesC decreases S. epidermidis biofilm 87
Ch
ap
ter
5.1
SE0383 iron-binding protein 295 Lipobox 9
SE1608 beta-lactamase 281 Lipobox 11
SE2320 lactococcal lipoprotein 281 Lipobox 11
SE0602 conserved hypothetical protein 270 Lipobox 11
SE1920 conserved hypothetical protein 267 Lipobox 9
SE0537 conserved hypothetical protein 266 PBD 7
SE1993 ABC transporter _periplasmic amino acid-binding protein_ 262 Lipobox 7
SE0142 conserved hypothetical protein 261 Lipobox 9
SE1852 modA protein 261 Lipobox 8
SE0145 conserved hypothetical protein 253 Lipobox 8
SE1480 sortase 253 PBD 7
SE0144 conserved hypothetical protein 251 Lipobox 7
SE0581 phage-related protein 248 Lipobox 7
SE0034 conserved hypothetical protein 227 Lipobox 9
SE1947 TpgX protein 215 Lipobox 7
SE0790 conserved hypothetical protein 208 Lipobox 7
SE2360 conserved hypothetical protein 207 Lipobox 5
SE2152 hypothetical protein 196 LPXTG 7
SE1215 conserved hypothetical protein 192 Lipobox 7
SE2342 conserved hypothetical protein 184 Lipobox 8
SE0569 conserved hypothetical protein 180 Lipobox 6
SE2221 conserved hypothetical protein 166 Lipobox 3
SE0467 conserved hypothetical protein 156 Lipobox 5
SE2029 conserved hypothetical protein 155 Lipobox 5
SE0481 conserved hypothetical protein 145 Lipobox 4
SE0447 conserved hypothetical protein 140 Lipobox 5
SE2399 hypothetical protein 133 Lipobox 6
SE0186 conserved hypothetical protein 132 Lipobox 4
SE1988 conserved hypothetical protein 122 Lipobox 4
SE0082 conserved hypothetical protein 117 Lipobox 5
SE1963 conserved hypothetical protein 116 Lipobox 4
SE1023 conserved hypothetical protein 77 Lipobox 1
Chapter
5.2
Mohammad ShahrooeiVishal Hira
Benoit StijlemansRita Merckx
Peter W. M. HermansJohan van Eldere
Infect Immun. 2009;77(9):3670-8
Inhibition of Staphylococcus epidermidis
biofilm formation by rabbit polyclonal
antibodies against the SesC protein
88
Chapter
5.2
Mohammad ShahrooeiVishal Hira
Benoit StijlemansRita Merckx
Peter W. M. HermansJohan van Eldere
Infect Immun. 2009;77(9):3670-8
Inhibition of Staphylococcus epidermidis
biofilm formation by rabbit polyclonal
antibodies against the SesC protein
Chapter 5.290
Abstract
Several well-studied proteins with defined roles in Staphylococcus epidermidis biofilm formation are LPXTG motif-containing proteins. Here, we investigate the possible use of the LPXTG motif-containing protein SesC (S. epidermidis surface protein C; accession no. NP_765787) as a target for antibodies to prevent biofilm formation. In vitro and in a in vivo rat model of catheter infection, gene and protein expression analysis showed that SesC is expressed more strongly in biofilm-associated cells than in planktonic cells and is expressed particularly during the late phase of in vivo biofilm formation. Polyclonal rabbit antibodies raised against SesC reduced the fibrinogen-binding ability of S. epidermidis RP62A and Staphylococcus aureus RN4220 transformants expressing SesC, inhibited in vitro biofilm formation by S. epidermidis strains 10b and 1457, and significantly reduced the numbers of bacteria in a 1-day-old in vivo biofilm (P < 0.001, one-way analysis of variance). Our findings revealed that SesC is a promising target for prevention and treatment of S. epidermidis biofilms because it affects both the primary attachment and biofilm accumulation phases. The precise role of SesC in biofilm formation remains to be identified.
Inhibition of S. epidermidis biofilm formation 91
Ch
ap
ter
5.2
Introduction
There has been substantial interest in Staphylococcus epidermidis in recent years as it is the most important cause of foreign-body infections (1, 2). Biofilm formation is a key factor in this process and is considered the most important virulence factor of S. epidermidis (3).S. epidermidis biofilm formation is a complex, multifactorial process, involving different factors that play roles at different stages in biofilm formation. Several of the genes that have been found to play important roles in biofilm formation by S. epidermidis (for a review, see reference (4)) encode LPXTG motif-containing proteins (Aap, Bhp, SdrF, and SdrG) (5-8). Recently, Söderquist reported that SesI, another LPXTG protein, was present in “about one-half” of the S. epidermidis isolates causing postoperative infection following cardiac surgery and might be a bacterial adherence factor (9).In publicly available genomes of S. epidermidis strains RP62A (10) and ATCC 12228 (11) 11 and 10 genes encoding LPXTG proteins, respectively, have been identified (12), including genes encoding the proteins mentioned above. Except for the five LPXTG proteins mentioned above, the roles of these LPXTG proteins have not been studied yet. In the present study we examined the S. epidermidis LPXTG protein SesC as a potential target for vaccination against S. epidermidis biofilms.Bowden et al. (12) reported that the sesC gene was present in all of the 116 clinical isolates of S. epidermidis that they investigated, indicating that it might be an essential gene. Yao et al. (13), however, reported that sesC was absent in some S. epidermidis isolates, particularly isolates from the skin of healthy individuals (9 of 20 isolates).SesC is predicted to encode a 676-amino-acid (aa) protein with a predicted molecular mass of 75 kDa. The cytoplasmic precursor of SesC contains a 35-aa N-terminal signal peptide (predicted using the SignalP server at http://www.cbs.dtu.dk/services/SignalP/), a 37-aa C-terminal LPXTG sorting signal, and a large extracellular domain. The N-terminal signal is required for sec-dependent secretion and is cleaved by signal peptidase. The C-terminal signal is needed for cleavage between the threonine and the glycine of the LPXTG motif and for attachment to peptidoglycan by sortase.The presence of mature SesC (±68 kDa) in the cell wall fraction of S. epidermidis RP62A in the exponential and stationary phases of growth was shown using a Western immunoblotting technique (12). All of the homologues of SesC in publicly available protein data banks had less than 70% sequence identity to SesC, and all of the homologues with identities higher than 26% were hypothetical proteins with unknown structures and functions. The closest homologue of SesC with a known function is a 341-aa fragment of clumping factor A (ClfA) (26.6% identity and 65.1% similarity in a 335-aa overlap). ClfA is a fibrinogen (Fg)-binding microbial surface component recognizing adhesive matrix molecules (MSCRAMM) of Staphylococcus aureus. However, the putative Fg-binding site of ClfA is located outside the similarity region.Targeting specific staphylococcal biofilm-associated factors is an alternative to treatment of staphylococcal infections with antibiotics (2). Antibodies against extracellular macromolecules and surface binding proteins essential for cell-surface and cell-cell interaction and adhesion, such as polysaccharide intracellular adhesin (PIA), teichoic acids, Fbe, and Aap, have been shown to prevent biofilm formation without killing the bacteria (14-17).In this study we demonstrate that SesC is highly expressed in biofilm-associated cells and we present data showing that it is a potential target for preventing S. epidermidis biofilm formation and for treating established mature biofilms with anti-SesC antibodies.
Chapter 5.292
Material & methods
Bacterial strains, plasmids, and mediaFor DNA manipulation and recombinant protein production, Escherichia coli strains DH5α and BL21(DE3), respectively, were used. Staphylococcus spp. were grown in brain heart infusion medium (Oxoid) or tryptone soya broth (TSB) (Oxoid), except where otherwise stated. E. coli was grown in Luria-Bertani medium. Solid media consisted of the liquid media supplemented with 1 to 2% agar. When required, antibiotics were added to the media as follows: chloramphenicol, 10 μg/ml for Staphylococcus spp.; erythromycin, 10 μg/ml for Staphylococcus spp. and 500 μg/ml for E. coli; tetracycline, 5 μg/ml for Staphylococcus spp. And E. coli; and ampicillin, 100 μg/ml for E. coli.The sesC sequence (SE2232; accession no. NP_765787) was retrieved from the National Center for Biotechnology Information from the complete genome of the non-biofilm-forming strain S. epidermidis ATCC 12228. Using the sesC sequence, primers and probes were designed with Primer Express 2.0 software (Applied Biosystems Division of Perkin-Elmer) and were purchased from Eurogentec (Seraing, Belgium). All fragments were PCR amplified using genomic DNA (gDNA) isolated from biofilm-forming S. epidermidis strain 10b (18). The primers and probe used are listed in Table 1.
A 388-bp fragment of the sesC gene in S. epidermidis 10b was PCR amplified using primers sesC-SF and sesC-SR. The amplicon was ligated into the pGEM-T Easy vector (Promega, Madison, WI), yielding pGEMsesC. Pure plasmid pGEMsesC was prepared and quantified as described previously (19, 20). Standard dilutions of a known quantity of pGEMsesC were used in real-time PCR.A 1,359-bp fragment of sesC in S. epidermidis 10b was amplified using primerssesC-RF and sesC-RR, which incorporate flanking NheI and BamHI restriction sites and a sequence coding
Table 1. Primers and probes used in this study.
Primer or probe DNA sequence (5′-3′)a
sesC-SF GTTGATAACCGTCAACAAGG
sesC-SR CATGTTGATCTTTTGAATCCC
sesC-TF AGCATCACCATCTAATAAAAACGAAA
sesC-TR CCATCATTACTTTTATCGTCTTTACTATCAC
sesC-P TAACAAAGAAGAATCTAGTACGACAACAAATCAATCCGA
sesC-RF ACGTGCTAGCGCAGATTCAGAAAGTACATC
sesC-RR GAACAGCTACAGCTGATCATCACCATCACCATCACTAGGATCCGCAT
16S rRNA-F TACACACCGCCCGTCACA
16S rRNA-R CTTCGACGGGCTAGCTCCAAAT
gmk-F AAGGTGCTAAGCAAGTAAGAAAGAAATT
gmk-P ATGCGTTGTTCATATTTTTAGCGCCTCCA
gmk-R CAACAAGACGTTCTTTCAAGTCATCT
sesC-EF TACGGGATCCCAGGTAACTTTATTAAAGGAGTATGTGTAA
sesC-ER ACGTGGTACCACTAGAAGTTAATGCAAGACCATCAATTTa Incorporated restriction sites are underlined.
Inhibition of S. epidermidis biofilm formation 93
Ch
ap
ter
5.2
for a C-terminal six-His tag, respectively. This fragment was cloned into a pET11c expression vector (Stratagene, La Jolla, CA), yielding pET11csesC, which was electrotransformed into E. coli BL21(DE3). The 1,359-bp fragment of sesC encodes a 459-aa extracellular part of SesC and contains a six-His tag at the C terminus. The truncated recombinant protein was used for immunization of rabbits.The entire coding region of the sesC gene of strain 10b was amplified using primers sesC-EF and sesC-ER, which incorporate flanking BamHI and KpnI restriction sites, respectively. The amplicon was ligated into the vectors pCN68 and pCN50 (21), yielding pCN68sesC and pCN50sesC. Plasmids pCN68, pCN50, pCN68sesC, and pCN50sesC were electroporated into S. aureus RN4220 (22), yielding strains RN-pCN68, RN-pCN50, RN-pCN68sesC, and RN-pCN50sesC, respectively. pCN50 and pCN50sesC were purified from RN4220 transformants and electroporated into S. epidermidis RP62A, yielding strains RP-pCN50 and RP-pCN50sesC, respectively. sesC gene expression in wild-type and transformed strains was quantified by TaqMan quantitative PCR.
Species identification and PCR screening for the sesC gene in clinical and commensal isolatesWe collected 239 coagulase-negative Staphylococcus spp. (CoNS) isolates from hospitalized patients (n = 215) or from the skin of healthy individuals (n = 24). Species were identified with Vitek 2 (bioMérieux). gDNA was extracted from each isolate using a Wizard gDNA purification kit (Promega) with addition of 30 μg/ml lysostaphin at the lysis step. A duplex PCR amplifying both sesC and the 16S rRNA genes was performed with all isolates. Primers for the 16S rRNA gene have been described previously (23).
Construction and purification of histidine-tagged fusion proteinA truncated recombinant SesC (rSesC) protein was expressed in E. coli BL21(DE3) using the expression vector pET11csesC as described previously (24). Briefly, after transformation, E. coli BL2(DE3) was grown with shaking (250 rpm) at 37°C in Luria-Bertani broth with 100 μg/ml ampicillin to an optical density at 600 nm (OD600) of 0.6 to 1.0. Expression was induced by addition of 1 mM isopropyl-β-D-thiogalactopyranoside (IPTG). After cooling on ice, cells were harvested by centrifugation (4,000 rpm, 10 min, 4°C), resuspended in 5 ml imidazole buffer (20 mM phosphate, 0.5 M NaCl, 10 mM imidazole), and frozen at −20°C. Then the preparation was sonicated three times for 30 s. After centrifugation (30 min, 15,000 rpm, 4°C) the supernatant was used for Ni+ affinity chromatography purification of the recombinant proteins with a HisTrap kit (Amersham Pharmacia, Uppsala, Sweden). The columns were washed with 40 mM imidazole buffer, and proteins were eluted with 300 mM imidazole buffer. The purified recombinant protein was dialyzed against 10 mM HEPES buffer (pH 7.5), freeze-dried, and stored at −20°C. The purity of the recombinant protein was determined by Coomassie blue staining of a sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel. The sequence of the purified peptide was confirmed by matrix-assisted laser desorption ionization-time of flight mass spectrometry.
Preparation and purification of polyclonal anti-SesC IgG antibodiesPolyclonal antibodies were produced by Eurogentec (Seraing, Belgium) by immunization of rabbits with purified rSesC protein. Total immunoglobulin Gs (IgGs) from preimmune serum and antisera directed against rSesC (anti-rSesC) were purified by absorption to a protein G column (GE Healthcare) according to the manufacturer’s instructions.
Chapter 5.294
In order to enrich for SesC-specific IgGs, (polyclonal anti-SesC IgGs), the rSecC antigen was covalently coupled to MiniLeak (medium) affinity resin (Kem-En-Tec, Copenhagen, Denmark) as recommended by the manufacturer. A total of 1 mg of rSecC was coupled to 1 ml of resin, and the coupling efficiency was measured as described by the manufacturer. Subsequently, 5 ml of purified IgG was incubated for 3 h at room temperature with 1 ml of immunoaffinity resin and was subsequently packed into a column. After the resin was washed with 100 ml of phosphate-buffered saline (PBS) (pH 6.8) containing 0.5 M NaCl and 10 mM EDTA, immunoabsorbed material was eluted with 0.1 M glycine-HCl buffer (pH 2.7) and immediately dialyzed against PBS. After dialysis the concentration was determined spectrophotometrically at 280 nm (an optical density of 1.4 was equivalent to 1 mg/ml). The purity of the IgGs was determined by Coomassie blue staining of sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels.The affinity of IgGs purified from preimmune serum and of polyclonal anti-SesC IgGs isolated from antiserum against rSesC was quantified with an alkaline phosphatase-conjugated anti-rabbit Ig by performing an indirect protein enzyme-linked immunosorbent assay using standard protocols.
In vitro and in vivo gene and protein expression studies The in vitro model and the in vivo rat model have been described previously (25-28). Slight modifications were incorporated into the anesthesia and euthanasia protocols for rats (19, 20) in accordance with guidelines of the animal ethics committee.In vivo and in vitro extraction of DNA and RNA from sessile and planktonic bacteria and reverse transcription were performed as described previously (19, 20, 23, 28).For in vitro studies, 20 μl of a frozen culture of S. epidermidis 10b was grown to the late exponential phase, pelleted, and resuspended in 0.9% NaCl. Seven-millimeter fragments of a commercial polyurethane intravenous catheter (Arrow International, Reading, PA) were added, and the mixture was incubated at 37°C. After various incubation periods, RNA and DNA were extracted as described previously (23, 25, 26, 28). Nucleic acid was isolated from bacteria adhering to the catheter fragments and from planktonic bacteria at zero time (n = 8) (just before the bacteria were suspended in 0.9% NaCl) and after 10 min (n = 16), 35 min (n = 16), 60 min (n = 16), 120 min (n = 16), and 180 min (n = 16). For all time points eight independent measurements from two experiments were included.For the in vivo rat model, first-generation descendants of inbred germfree Fisher rats were used. These rats were exposed to normal rat flora from birth and were designated ex-germfree Fisher rats. Seven-millimeter catheter fragments were inoculated with a small amount of S. epidermidis 10b (1.09 × 104 cells/catheter) prior to implantation by incubation for 20 min at 37°C in a 0.9% NaCl suspension of S. epidermidis. This resulted in a 100% infection rate. Rats were anesthetized by inhalation of enflurane gas (Alyrane; Pharmacia). Rats were kept asleep during the implantation procedure by using a mixture of enflurane (20%) and oxygen (80%). A large area on the back of each rat was shaved, and the skin was disinfected with 0.5% chlorhexidine in 70% alcohol and allowed to dry. A 10-mm incision was made at the base of the tail, and the subcutis was dissected to create three subcutaneous tunnels. For each rat, eight catheter fragments were inserted at least 2 cm from the incision; the distance between any two fragments was at least 1 cm.For catheter explantation, rats were euthanized by CO2 inhalation. The skin was disinfected before removal of the catheter fragments. All catheter fragments from the same animal were used for a single time point. In each experiment, baseline levels of gene expression
Inhibition of S. epidermidis biofilm formation 95
Ch
ap
ter
5.2
in sessile bacteria before implantation were determined (time zero; n = 16). A total of 176 polyurethane catheter segments were implanted and explanted at 11 different time points. The time points used were 15 min (n = 16), 1 h (n = 16), 2 h (n = 16), 4 h (n = 16), 6 h (n = 16), 12 h (n = 16), 24 h (n = 16), 2 days (n = 16), 4 days (n = 16), 7 days (n = 16), and 14 days (n = 16). Data for each in vivo time point were obtained from 16 independent measurements obtained in two independent experiments. Nucleic acid isolation and cDNA synthesis were performed immediately after explantation as described previously (23, 28).Using the TaqMan primers sesC-TF and sesC-TR and a dual-label probe (5′ 6-carboxyfluorescein and 3′ 6-carboxytetramethylrhodamine), sesC-P quantification of both cDNA and gDNA was performed as described previously (19, 20, 23, 28). In each run, a standard dilution of the plasmid (pGEMsesC) with a known quantity that allowed gene quantification and a negative control (distilled water) were included.Conventional fluorescence microscopy was used to confirm SesC protein expression in sessile and planktonic cells in vitro. The method used for preparation of samples for fluorescence microscopy was similar to the method used for preparation of samples for gene expression analysis in vitro, except that samples were taken at different time points (0, 30, 60, 90, and 120 min) after inoculation and sessile cells were separated from catheter fragments as described previously (20). The bacteria were fixed using PBS containing 1.5% formaldehyde and 0.5% glutaraldehyde for 30 min and were washed with PBS. Next, the bacteria were preincubated for 20 min on ice with 2.4G2 (Fc-blocking antibody; BD Pharmingen), after which anti-SesC IgGs were added at a concentration of 5 μg per 100 μl and the preparations were incubated for another 30 min. Subsequently, the bacteria were washed twice with ice-cold PBS, and fluorescein isothiocyanate (FITC)-labeled goat anti-rabbit antibody (BD-Pharmingen) was added for 30 min. Finally, the cells were washed twice with PBS and viewed with a fluorescence microscope (Leica, Germany) equipped with an oil immersion Plan Neofluar objective (×100; numeric aperture, 1.25).The following preparations were used as negative controls: (i) cells incubated with preimmune IgGs and FITC-labeled goat anti-rabbit, (ii) cells incubated with polyclonal rabbit antibody against mouse Igs and FITC-labeled goat anti-rabbit, and (iii) cells incubated with only FITC-labeled goat anti-rabbit.
In vitro and in vivo biofilm inhibition assaysThe effect of IgGs (preimmune or anti-SesC) on in vitro biofilm formation during the first hour (primary attachment) and overnight (14 h) and on 1-day-old biofilms was studied as described previously (16, 29). For quantification of biofilms, 20-μl portions of frozen cultures of S. epidermidis strains 10b and 1457 (30) and a biofilm-forming sesC-negative clinical isolate of S. warneri (this study) were inoculated into 5 ml TSB and grown to the late exponential phase in a shaking incubator at 37°C. Cultures were subsequently diluted to an OD600 of 0.005 (5 × 106 CFU/ml) in fresh TSB.To evaluate the effect of IgGs on primary attachment of S. epidermidis strains 10b and 1457, starting cultures were diluted to an OD600 of 0.005 and subsequently grown at 37°C to an OD600 of 1. Cultures then were mixed with either preimmune IgGs or anti-SesC IgGs (10 μg/ml), and after 2 h of incubation at 4°C, 200-μl portions of the mixtures were pipetted into 96-well polystyrene microtiter plates (BD Biosciences, Heidelberg, Germany) and incubated for 1 h at 37°C without shaking.To study the effect of IgGs on biofilm formation for 14 h, cultures diluted to an OD600 of 0.005 were mixed with either preimmune IgGs or anti-SesC IgGs at concentrations of 1 to 4 μg/
Chapter 5.296
ml. The mixtures were incubated for 2 h at 4°C. Two hundred microliters of a mixture (106 cells per well) was added to each well of 96-well polystyrene microtiter plates and incubated overnight at 37°C without shaking.To evaluate the effect of IgGs on 1-day-old biofilms of strains 10b and 1457, diluted overnight cultures of bacteria with an OD600 of 0.005 were pipetted into sterile 96-well polystyrene microtiter plates. Twenty-four hours later, the growth medium was replaced with fresh medium or fresh medium containing 5 μg/ml preimmune IgGs or anti-SesC IgGs and incubated at 37°C for 24 h without shaking.After this incubation, the plates were washed three times with PBS, and adherent biofilms were stained with 200 μl of 1% (wt/vol) crystal violet (Sigma) for 10 min, after which the plates were washed three times with water and dried. For quantification, 160 μl of 30% (vol/vol) acetic acid was added to each well to dissolve the stain. The OD595 of the dissolved stain was measured with a multipurpose UV/visible plate reader. The average adherence with each concentration of IgGs was determined using at least eight independent measurements obtained in at least two independent experiments. S. epidermidis strains 10b and 1457 and the clinical isolate of S. warneri in TSB without any added IgGs were used as positive controls, and TSB without bacteria was used as a negative control.For the in vivo inhibition assay, our rat model for in vivo catheter infection was used. Seven-millimeter catheter fragments, preincubated for 20 min at 37°C with S. epidermidis 10b before implantation, were placed on ice, and eight fragments per rat were implanted immediately in ex-germfree Fisher rats. After 24 h, the rats (nine rats divided into three groups of three rats) were treated with 50 μg of anti-SesC IgG diluted in PBS (total volume, 330 μl), 50 μg of preimmune IgG diluted in PBS (total volume, 330 μl), or 330 μl of PBS via a subcutaneous injection at the place of catheter insertion.Twenty-four hours after injection, all eight catheter fragments from each rat were explanted and used for nucleic acid extraction as described previously (23, 28). Real-time quantitative PCR of the guanylate monokinase housekeeping gene (gmk; SE0885; accession no. NP_764440) was used to determine the number of bacteria attached to the catheter fragments. As previously demonstrated (28), the number of gmk copies per catheter correlates very well with the number of CFU per catheter.
Adherence of transformants to immobilized Fg, Fn, Cn, and VWF in the presence and absence of anti-SesC IgGsOvernight cultures in brain heart infusion medium of transformed strains and their parental strains were precipitated and washed once with PBS. The OD600 was adjusted to 1.0, and the adherence was measured as follows. Wells of polystyrene microtiter plates were coated with human Fg (Sigma), fibronectin (Fn) (Sigma), collagen (Cn) (Sigma), and Von Willebrand factor (VWF) (Sigma) in PBS overnight at concentrations ranging from 0.1 to 100 μg/ml. Blocking was done with 2% bovine serum albumin in PBS for 1 h at 37°C. After washing, either the pure cultures (100 μl per well) were pipetted into the plates or the cultures were mixed with 5 μg/ml (final concentration) preimmune IgGs or anti-SesC IgGs and after 2 h of incubation at 4°C were added to the plates and allowed to adhere to the coated surfaces for 2 h at 37°C. After the incubation period, culture supernatants were washed, and the remaining adherent cells were stained and quantified as described above.
Inhibition of S. epidermidis biofilm formation 97
Ch
ap
ter
5.2
Statistical analysisAll statistical analyses of the in vitro and in vivo gene expression data were performed with GraphPad Prism (GraphPad software, version 4.2; GraphPad, San Diego, CA) as described previously (19, 20, 23, 28). Since the in vitro and in vivo cDNA/gDNA ratios were not normally distributed at any time point, all data were log10 transformed in order to fulfill the requirements of normality.For the in vitro gene expression data, two hypotheses were tested. A significant change in gene expression levels over time within one group (sessile or planktonic) was tested using a one-way analysis of variance (ANOVA). A significant difference in the evolution over time of the gene expression levels between the sessile group and the planktonic group was tested using a two-way ANOVA. When the one-way ANOVA result was significant, two-sided univariate tests with a correction for multiple comparisons were performed (Bonferroni test) to locate the significant differences.For the in vivo gene expression data, a one-way ANOVA was used to test if there was significant change in the expression levels over time. When the one-way ANOVA result was significant, the two-sided Bonferroni multiple-comparison method was used to determine which time points differed at α = 0.05, with a correction for multiple comparisons.For all data from bacterial adherence assays, two hypotheses were tested. A significant change in the adherence levels at different concentrations of IgGs within one group (preimmune or anti-SesC IgGs) was tested using a one-way ANOVA. A significant difference in the adherence at different concentrations of IgGs between the preimmune IgGs and anti-SesC IgG groups was tested with a two-way ANOVA. When the one-way ANOVA result was significant, two-sided univariate tests with a correction for multiple comparisons were performed (Bonferroni test) to locate the significant differences.
Results
Presence of sesC in S. epidermidis and non-S. epidermidis coagulase-negative Staphylococcus spp Of 239 isolates from patients and healthy persons, 105 were identified as S. epidermidis and 134 isolates were identified as other CoNS, including S. hominis (n = 17), S. haemolyticus (n = 58), S. warneri (n = 43), S. capitis (n = 15), and S. saprophyticus (n = 1). All 105 S. epidermidis isolates were sesC positive, whereas the non-S. epidermidis isolates were either sesC negative (80%) or sesC positive (20%).
SesC gene and protein expression in sessile and planktonic bacteria in vitro and in sessile bacteria in vivoBiofilm-associated bacteria have been shown to have low metabolic activity and altered gene expression patterns (26, 27) To confirm expression of sesC in sessile and planktonic bacteria, we analyzed gene and protein expression levels.In our in vitro model, in sessile as well as planktonic bacteria, expression of sesC decreased 10-fold during the first 35 min after inoculation (P < 0.01, one-way ANOVA) and then remained at an intermediate level until the end of the experiment (at 180 min) in planktonic bacteria. In sessile bacteria, expression of this gene increased 13-fold from 35 min to 60 min (P < 0.001, one-way ANOVA) and then remained at this increased level for the remainder of the 2-h observation period (Figure 1). However, the difference in sesC gene expression
Chapter 5.298
between sessile and planktonic bacteria was not statistically significant (P > 0.05, two-way ANOVA).To confirm the results obtained in the gene expression analysis, purified polyclonal anti-SesC IgGs and FITC-labeled goat anti-rabbit antibodies were used in an immunofluorescence assay to study in vitro protein expression. Comparison of the fluorescence microscopy images of sessile and planktonic bacteria obtained at different time points confirmed that SesC was present in both sessile and planktonic bacteria. Visual comparison of images taken at different time points suggested that there was a higher level of fluorescence in sessile bacteria than in planktonic bacteria (Figure 1). However, no quantitative measurements were obtained.
After implantation of catheters with sessile bacteria in our rat model, a 1.336-log10 or 21-fold increase in sesC expression in the sessile bacteria was observed after 2 h. The expression peaked at 4 h (1.47-log10 or 34-fold increase, a significant change over time [P < 0.001, one-way ANOVA]). This increase was followed by a 2.026-log10 or 106-fold decrease in gene expression in the next 20 h (P < 0.001, one-way ANOVA). However, after 24 h, gene expression again increased up to a maximum value at 168 h (P < 0.001, one-way ANOVA) and was again lower at 336 h after implantation (P < 0.05, one-way ANOVA) (Figure 2).
-3
-2.5
-2
-1.5
-1
-0.5
0
0 30 60 90 120 150 180
Time (min)
Log1
0 cD
NA
/ gDN
A
sesC_Planktonic
sesC_Sessile
Figure 1. In vitro sesC gene expression and extracellular presence of the SesC protein in planktonic and sessile bacteria. Gene expression is expressed as the log10 cDNA/gDNA ratio. The error bars indicate standard deviations. At each time point eight samples from two independent experiments were assessed. The fluorescence microscopy images are images of sessile bacteria (top images on the right) and planktonic bacteria (bottom images on the right) at different time points. Images A1 and A2 show bacterial cells plus preimmune IgGs and FITC-labeled goat anti-rabbit antibodies. Images B1 and B2 are images of control samples containing bacterial cells plus polyclonal rabbit antibody against mouse Igs and FITC-labeled goat anti-rabbit antibodies. Images C1 and C2 are images of control samples containing bacterial cells plus only FITC-labeled goat anti-rabbit antibodies. Images A1, B1, and C1 are fluorescence images, while images A2, B2, and C2 are bright-field images.
Inhibition of S. epidermidis biofilm formation 99
Ch
ap
ter
5.2
Biofilm inhibition by purified preimmune IgGs and polyclonal anti-SesC IgGsBinding of the purified preimmune IgGs and of the anti-SesC IgGs was tested by performing an indirect enzyme-linked immunosorbent assay with the 65-kDa rSesC. Tests performed with increasing concentrations of purified preimmune IgGs and anti-SesC IgGs showed that there was no binding of the preimmune IgGs and that there was dose-dependent binding of the purified anti-SesC IgGs (data not shown).Different modes of preincubation of bacteria with IgGs (preimmune IgGs or polyclonal anti-SesC IgGs) and of incubation of bacteria and IgGs in 96-well polystyrene microtiter plates were tested in our in vitro model for biofilm formation.Preincubation of strains 10b and 1457 for 2 h with anti-SesC IgGs or preimmune IgGs at 4°C and subsequent incubation for 1 h in polystyrene wells led to a significant reduction in initial attachment compared to controls for anti-SesC IgGs (P < 0.001, one-way-ANOVA) but not for preimmune IgGs (Figure 3).
-3,5
-3
-2,5
-2
-1,5
-1
-0,5
0
0 0,25 1 2 4 6 12 24 48 96 168 336
Time (h)
sesC
Log1
0 cD
NA/g
DNA
* §
*
§
§
§
*
Figure 2. Levels of sesC expression over time in vivo for 2 weeks after implantation. The expression level is expressed as the log10 cDNA/gDNA ratio. The error bars indicate standard deviations. At each time point 16 samples from two independent experiments were assessed. Significant differences for time points compared to time zero and previous time points are indicated by asterisks and section signs, respectively.
Figure 3. Effect of anti-SesC IgGs on primary attachment of S. epidermidis 10b and 1457 to polystyrene surfaces.Bacteria were mixed with IgGs, incubated for 2 h at 4°C, and then pipetted into wells. After 1 h of incubation at 37°C, the plates were washed and stained with crystal violet, and the OD595 was measured. The error bars indicate standard deviations. The data are the averages of eight measurements obtained in two independent experiments.
Chapter 5.2100
Two hours of preincubation of bacteria with IgGs followed by overnight (14-h) incubation in the 96-well polystyrene microtiter plates showed that the polyclonal anti-SesC IgGs purified from rSesC-induced rabbit antisera exhibited dose-dependent S. epidermidis biofilm inhibition activity, whereas the purified IgGs from preimmune serum showed only low activity that was dose independent (Figure4). Increasing the concentration of polyclonal anti-SesC IgGs from 1 to 4 μg/ml increased the inhibition of S. epidermidis strain 10b and 1457 biofilms from 40 to 70% (P < 0.001, one-way-ANOVA) (Figure 4A and B). For both strains, the inhibition effects seen with anti-SesC IgGs were significantly different from those observed with preimmune IgGs (P < 0.001, two-way-ANOVA). A sesC-negative biofilm-positive S. warneri strain was included as a control; for this strain the inhibitory effect of anti-SesC IgGs was not different from that of the preimmune serum and was not dose dependent (Figure 4C). For all three strains, the effect of preimmune IgGs on biofilm formation was not significant and was dose independent (P > 0.05, one-way ANOVA).
The effect of anti-SesC IgGs on 1-day-old biofilms of strain 10b was significant (P < 0.001, one-way ANOVA) compared to the effect on nontreated biofilms or biofilms treated with preimmune IgGs (Figure 5). However, anti-SesC IgGs had no effect on 1-day-old biofilms of S. epidermidis strain 1457 (data not shown).
Figure 4. Inhibition of biofilm formation by S. epidermidis strains 10b (A) and 1457 (B) and a sesC-negative biofilm-positive clinical isolate of S. warneri (C) with increasing concentrations of preimmune and anti-SesC IgGs. Overnight cultures were diluted to an OD600 of 0.005, mixed with the indicated concentrations of IgGs, and incubated for 2 h at 4°C and then overnight at 37°C. The formation of biofilms was measured using the stain crystal violet. The error bars indicate standard errors. For each concentration of IgGs at least nine independent measurements from three independent experiments were assessed. One hundred percent adherence was defined as biofilm formation in TSB without any IgG.
Inhibition of S. epidermidis biofilm formation 101
Ch
ap
ter
5.2
In the in vivo rat model, the number of sessile bacteria was determined after 1 day by quantification of the gDNA copies of the housekeeping gene gmk. Injection of 50 μg preimmune IgGs reduced the number of biofilm-associated S. epidermidis10b bacteria 0.64 log10 or 4-fold (P < 0.01, one-way ANOVA), whereas injection of 50 μg anti-SesC IgGs decreased the number of biofilm-associated bacteria 1.78 log10 or 60.42-fold and 1.15 log10 or 14.35-fold (P < 0.001, one-way ANOVA) compared to the control group treated with PBS and the group treated with preimmune IgG, respectively (Figure 6).
Figure 5. Effect of anti-SesC IgGs on 1-day-old biofilms of S. epidermidis 10b on polystyrene surfaces in vitro. After 24 h of incubation at 37°C the growth media were replaced with fresh TSB (control) or TSB containing 5 μg/ml preimmune or anti-SesC IgGs and then incubated for 24 h at 37°C. The remaining biofilms were stained with crystal violet and quantified by determining the OD595. The error bars indicate standard deviations. The data are the averages of eight measurements obtained in two independent experiments.
4,5
5
5,5
6
6,5
7
7,5
Control (PBS) Pre-Immune IgG SesC-IgG
log1
0 (g
DN
A co
pies
gm
k)
P<0.01
P <0.001
P<0.001
Figure 6. Effect of anti-SesC IgGs on 1-day-old biofilms of S. epidermidis 10b in vivo. One-day-old biofilms on catheter fragments implanted in three groups of rats (three rats per group) were treated with 50 μg preimmune IgGs or anti-SesC IgGs diluted in PBS (total volume, 330 μl) or with PBS via subcutaneous injection at the site of catheter insertion. The next day, catheter fragments were explanted, and the number of sessile bacteria was determined by quantification of the gDNA copies of the housekeeping gene gmk. The error bars indicate standard deviations. The data for each group were obtained using bacteria adhering to 24 catheter fragments implanted in three rats.
Chapter 5.2102
SesC is a potential Fg-binding MSCRAMMTo explore the function of SesC, we expressed SesC in the sesC-negative strain S. aureus RN4220 and overexpressed SesC in the sesC-positive strain S. epidermidis RP62A by transformation of these strains with pCN68sesC and pCN50sesC. The copy number of pCN68 in S. aureus is higher than that of pCN50. RN transformants expressing SesC (RN-pCN68sesC and RN-pCN50sesC) and the RP transformant RP-pCN50sesC exhibited greater Fg-binding ability than their parental strains (Figure 7). The levels of binding of these transformants to other host extracellular matrix (ECM) proteins (Fn, Cn, and VWF) were similar to the levels of binding of their parental strains (data not shown). RN-pCN68sesC had significantly greater Fg-binding ability than RN4220 and RN-pCN68 (P < 0.001 and P < 0.01, one-way ANOVA). Compared to their parental strains, RN-pCN50sesC and RP-pCN50sesC also exhibited significantly greater Fg-binding ability (P < 0.05 and P < 0.01, respectively, one-way ANOVA). However, the Fg-binding abilities of RN-pCN50sesC and RP-pCN50sesC were not significantly different from those of their parental strains transformed by mock plasmids (RN-pCN50 and RP-pCN50). There was no significant difference between the Fg-binding levels of RN4220 and RN-pCN50 or between the Fg-binding levels of RP62A and RP-pCN50.Addition of anti-SesC IgGs reduced the Fg-binding abilities of RN-pCN68sesC and RN-pCN50sesC to the wild-type strain levels and also reduced the Fg-binding abilities of wild-type and transformed RP strains (Figure 7).Anti-SesC IgGs had no effect on the abilities of RN and RP wild-type strains and transformed strains to bind to Fn, Cn, and VWF (data not shown).
Discussion
Primary attachment of S. epidermidis to unmodified polymer surfaces is mediated by several protein and carbohydrate factors, such as PS/A, AtlE, and surface proteins SSP-1 and -2 (4). In vivo medical devices are quickly coated with adsorbed blood plasma proteins that can interact with S. epidermidis surface factors and promote binding. Once attached, S. epidermidis proliferates and accumulates as multilayer cell clusters. In addition to PIA, proteins are also important for biofilm formation. Therefore, antibodies directed against S. epidermidis surface targets involved in attachment or accumulation could be interesting alternatives to current antibiotic-dependent treatment of biofilms.
Figure 7. Fg-binding abilities of RN4220 (A) and RP62A (B) transformants in the presence and absence of anti-SesC IgG. The abilities of strains RN4220 and RP62A and their transformants to bind immobilized Fg were measured using plates coated with Fg. The binding activities of RN and RP strains were determined on plates coated with Fg at concentrations of 10 and 1 μg/ml, respectively.
Inhibition of S. epidermidis biofilm formation 103
Ch
ap
ter
5.2
Here we show that SesC is an extracellular protein that is expressed more in sessile bacteria than in planktonic bacteria and that antibodies targeted against SesC reduce biofilm formation on polystyrene microtiter plates in vitro and on subcutaneously implanted polyurethane catheters in vivo.In our gene expression analysis, we observed that in vitro and in vivo adherence to polyurethane catheters did not sharply increase sesC expression. The initial adherence takes place very rapidly after inoculation and is usually complete within the first hour. Upregulation of sesC gene expression occurred after 1 h in vitro, after 2 h in vivo, and during the late stages of in vivo foreign-body infections. This appears to indicate that SesC is associated more with the accumulation phase and with persistence of a biofilm than with the initial adhesion. This is consistent with the fluorescence data. The fact that more SesC was present on the surface of sessile cells than on planktonic cells suggests that SesC has a specific function in biofilm formation.The precise function of SesC is not known. We found that the sesC gene was present in all S. epidermidis strains but was not universally present in non-S. epidermidis CoNS isolates. All sesC-negative non-S. epidermidis CoNS strains were biofilm negative, except a few (3 of 107) agr-negative isolates that were biofilm positive. The agr quorum-sensing system is one of the global regulators in Staphylococcus spp. that have important functions in the regulation of the biofilm phenotype. In S. epidermidis, agr represses biofilm formation (31). On the other hand, sesC-positive non-S. epidermidis CoNS can be either biofilm positive or biofilm negative. This observation suggests that sesC might code for an essential function in S. epidermidis biofilm formation.So far, we have failed to knock out sesC in S. epidermidis, and we have not found a natural S. epidermidis sesC mutant. Expression of sesC in the sesC-negative strain S. aureus RN4220 or overexpression of sesC in the sesC-positive strain S. epidermidis RP62A did have a clear effect on Fg binding but not on binding to other host ECM proteins. This finding suggests that SesC can act as an Fg-binding MSCRAMM. However, the truncated rSesC did not show any Fg-binding activity (data not shown).Several recent studies have shown that application of antibodies against surface components of S. epidermidis can affect the rate of biofilm formation or the adherence of bacteria to medical devices in vitro. Cerca et al. showed that antibodies against PIA readily penetrated a biofilm and bound to the sessile cells (32). Sessile bacteria were, however, more resistant to opsonic killing than their planktonic counterparts. Using polyclonal antibodies against Fbe, Pei and Flock blocked adherence of S. epidermidis to Fg-coated catheters in vitro (15). Sun et al. showed that monoclonal antibodies against AAP can significantly reduce the accumulation phase during biofilm formation by S. epidermidis in vitro (16).Our in vitro experiments showed that rabbit polyclonal anti-SesC IgGs could significantly reduce primary attachment of S. epidermidis to the abiotic and Fg-coated surfaces of polystyrene plates and inhibit biofilm formation by S. epidermidis strains 10b and 1457 to less than 40% of control in a dose-dependent manner. In contrast, the effect of rabbit polyclonal anti-SesC IgGs on primary attachment of S. epidermidis to the surfaces of plates coated with other host ECM proteins or on biofilm formation by a sesC-negative biofilm-positive S. warneri strain was not significant, dose independent, and limited. Anti-SesC IgGs could also affect 1-day-old biofilms of strain 10b on polystyrene microtiter plates in vitro and on subcutaneously implanted polyurethane catheters in vivo.Different explanations are possible. It is possible that in vivo opsonic activity plays a role in addition to the direct effect of antibody binding on the activity of SesC, as Rennermalm et
Chapter 5.2104
al. have previously described for Fbe (33). Other workers have shown that antigen-antibody binding may inhibit ligand-receptor interactions or may lead to conformational changes (34-36). The inhibition of biofilm formation by preimmune IgGs could be due to cross-reactive IgGs directed against other surface-exposed antigens of Staphylococcus spp.Gene expression data support the conclusion that SesC is involved in the accumulation phase and persistence of biofilms, whereas biofilm inhibition studies show that this protein is involved in primary attachment to naked and Fg-coated surfaces of polystyrene microtiter plates as well. An example of a protein which plays a role in attachment to abiotic surfaces and also shows host matrix protein-binding activity is AtlE (37).Anti-SesC IgGs could restore the Fg-binding level of the RN transformants to the Fg-binding level of their parental strain and significantly reduce the Fg-binding ability of RP62A and its transformants, but they had no effect on the Cn-, Fn-, and VWF-binding abilities of the strains tested. These data indicate that anti-SesC IgGs efficiently and specifically inhibit the SesC function and have no effect on the function of other MSCRAMMs.In conclusion, the effect of antibodies against SesC on S. epidermidis biofilm formation suggests that SesC might be a promising target for inhibition of S. epidermidis biofilm formation. The expression of SesC at the gene and protein levels in sessile S. epidermidis is in line with the biofilm inhibition data and support a role for SesC in S. epidermidis biofilm formation. The sesC gene is present in all biofilm-positive and –negative S. epidermidis isolates, suggesting that SesC may be a factor whose presence is necessary but not sufficient for biofilm formation.
Acknowledgements
This work was supported by the Glaxo-Wellcome Chair in Medical Microbiology (J. Van Eldere) at the Catholic University of Leuven (Leuven, Belgium), by the Sophia Foundation for Medical Research, and by the Erasmus MC—Sophia Children’s Hospital, Rotterdam, The Netherlands.We thank Caroline Massonet, Valerie Pintens, Marcel Sluijter, Theo Hoogeboezem, and Gholamreza Hassanzadeh Ghassabeh for their excellent assistance.
Inhibition of S. epidermidis biofilm formation 105
Ch
ap
ter
5.2
References
1. Vadyvaloo V, Otto M. Molecular genetics of Staphylococcus epidermidis biofilms on indwelling medical devices. The International journal of artificial organs. 2005;28:1069-1078.
2. Vuong C, Otto M. Staphylococcus epidermidis infections. Microbes and infection / Institut Pasteur. 2002;4:481-489.
3. Christensen GD, Simpson WA, Bisno AL, Beachey EH. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun. 1982;37:318-326.
4. Otto M. Virulence factors of the coagulase-negative staphylococci. Frontiers in bioscience : a journal and virtual library. 2004;9:841-863.
5. Arrecubieta C, Lee MH, Macey A, Foster TJ, Lowy FD. SdrF, a Staphylococcus epidermidis surface protein, binds type I collagen. J Biol Chem. 2007;282:18767-18776.
6. Cucarella C, Solano C, Valle J, et al. Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J Bacteriol. 2001;183:2888-2896.
7. Davis SL, Gurusiddappa S, McCrea KW, Perkins S, Hook M. SdrG, a fibrinogen-binding bacterial adhesin of the microbial surface components recognizing adhesive matrix molecules subfamily from Staphylococcus epidermidis, targets the thrombin cleavage site in the Bbeta chain. J Biol Chem. 2001;276:27799-27805.
8. Hussain M, Herrmann M, von Eiff C, Perdreau-Remington F, Peters G. A 140-kilodalton extracellular protein is essential for the accumulation of Staphylococcus epidermidis strains on surfaces. Infect Immun. 1997;65:519-524.
9. Soderquist B. Surgical site infections in cardiac surgery: microbiology. APMIS. 2007;115:1008-1011.10. Gill SR, Fouts DE, Archer GL, et al. Insights on evolution of virulence and resistance from the complete genome
analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J Bacteriol. 2005;187:2426-2438.
11. Zhang YQ, Ren SX, Li HL, et al. Genome-based analysis of virulence genes in a non-biofilm-forming Staphylococcus epidermidis strain (ATCC 12228). Mol Microbiol. 2003;49:1577-1593.
12. Bowden MG, Chen W, Singvall J, et al. Identification and preliminary characterization of cell-wall-anchored proteins of Staphylococcus epidermidis. Microbiology. 2005;151:1453-1464.
13. Yao Y, Sturdevant DE, Villaruz A, et al. Factors characterizing Staphylococcus epidermidis invasiveness determined by comparative genomics. Infect Immun. 2005;73:1856-1860.
14. McKenney D, Pouliot K, Wang Y, et al. Vaccine potential of poly-1-6 beta-D-N-succinylglucosamine, an immunoprotective surface polysaccharide of Staphylococcus aureus and Staphylococcus epidermidis. Journal of biotechnology. 2000;83:37-44.
15. Pei L, Flock JI. Functional study of antibodies against a fibrogenin-binding protein in Staphylococcus epidermidis adherence to polyethylene catheters. J Infect Dis. 2001;184:52-55.
16. Sun D, Accavitti MA, Bryers JD. Inhibition of biofilm formation by monoclonal antibodies against Staphylococcus epidermidis RP62A accumulation-associated protein. Clin Diagn Lab Immunol. 2005;12:93-100.
17. Williams RJ, Henderson B, Sharp LJ, Nair SP. Identification of a fibronectin-binding protein from Staphylococcus epidermidis. Infect Immun. 2002;70:6805-6810.
18. Van Wijngaerden E, Peetermans WE, Vandersmissen J, et al. Foreign body infection: a new rat model for prophylaxis and treatment. J Antimicrob Chemother. 1999;44:669-674.
19. Massonet C, Pintens V, Merckx R, et al. Effect of iron on the expression of sirR and sitABC in biofilm-associated Staphylococcus epidermidis. BMC microbiology. 2006;6:103.
20. Pintens V, Massonet C, Merckx R, et al. The role of sigmaB in persistence of Staphylococcus epidermidis foreign body infection. Microbiology. 2008;154:2827-2836.
21. Charpentier E, Anton AI, Barry P, et al. Novel cassette-based shuttle vector system for gram-positive bacteria. Appl Environ Microbiol. 2004;70:6076-6085.
22. Kreiswirth BN, Lofdahl S, Betley MJ, et al. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983;305:709-712.
23. Vandecasteele SJ, Peetermans WE, Merckx R, Van Eldere J. Quantification of expression of Staphylococcus epidermidis housekeeping genes with Taqman quantitative PCR during in vitro growth and under different conditions. J Bacteriol. 2001;183:7094-7101.
24. Hermans PW, Adrian PV, Albert C, et al. The streptococcal lipoprotein rotamase A (SlrA) is a functional peptidyl-prolyl isomerase involved in pneumococcal colonization. J Biol Chem. 2006;281:968-976.
25. Goerke C, Fluckiger U, Steinhuber A, Zimmerli W, Wolz C. Impact of the regulatory loci agr, sarA and sae of Staphylococcus aureus on the induction of alpha-toxin during device-related infection resolved by direct quantitative transcript analysis. Mol Microbiol. 2001;40:1439-1447.
26. Vandecasteele SJ, Peetermans WE, Carbonez A, Van Eldere J. Metabolic activity of Staphylococcus epidermidis is high during initial and low during late experimental foreign-body infection. J Bacteriol. 2004;186:2236-2239.
27. Vandecasteele SJ, Peetermans WE, Merckx R, Van Eldere J. Expression of biofilm-associated genes in
Chapter 5.2106
Staphylococcus epidermidis during in vitro and in vivo foreign body infections. J Infect Dis. 2003;188:730-737.28. Vandecasteele SJ, Peetermans WE, Merckx R, Van Ranst M, Van Eldere J. Use of gDNA as internal standard
for gene expression in staphylococci in vitro and in vivo. Biochem Biophys Res Commun. 2002;291:528-534.29. Christensen GD, Simpson WA, Younger JJ, et al. Adherence of coagulase-negative staphylococci to plastic
tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985;22:996-1006.
30. Mack D, Siemssen N, Laufs R. Parallel induction by glucose of adherence and a polysaccharide antigen specific for plastic-adherent Staphylococcus epidermidis: evidence for functional relation to intercellular adhesion. Infect Immun. 1992;60:2048-2057.
31. Vuong C, Gerke C, Somerville GA, Fischer ER, Otto M. Quorum-sensing control of biofilm factors in Staphylococcus epidermidis. J Infect Dis. 2003;188:706-718.
32. Cerca N, Jefferson KK, Oliveira R, Pier GB, Azeredo J. Comparative antibody-mediated phagocytosis of Staphylococcus epidermidis cells grown in a biofilm or in the planktonic state. Infect Immun. 2006;74:4849-4855.
33. Rennermalm A, Nilsson M, Flock JI. The fibrinogen binding protein of Staphylococcus epidermidis is a target for opsonic antibodies. Infect Immun. 2004;72:3081-3083.
34. Brown JC, Koshland ME. Evidence for a long-range conformational change induced by antigen binding to IgM antibody. Proceedings of the National Academy of Sciences of the United States of America. 1977;74:5682-5686.
35. Einhauer A, Jungbauer A. Complex formation of a calcium-dependent antibody: a thermodynamical consideration. Journal of chromatography A. 2003;1009:81-87.
36. Oda M, Kozono H, Morii H, Azuma T. Evidence of allosteric conformational changes in the antibody constant region upon antigen binding. International immunology. 2003;15:417-426.
37. Heilmann C, Hussain M, Peters G, Gotz F. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol Microbiol. 1997;24:1013-1024.
Chapter
6
Vishal HiraRené F. Kornelisse
Ronald de GrootPeter W. M. Hermans
Submitted
Prevention of coagulase-negative staphylococcal
late-onset sepsisin neonates
108
Chapter
6
Vishal HiraRené F. Kornelisse
Ronald de GrootPeter W. M. Hermans
Submitted
Prevention of coagulase-negative staphylococcal
late-onset sepsisin neonates
Chapter 6110
Abstract
Coagulase-negative staphylococci (CoNS) are the most important pathogens in late-onset sepsis (LOS) at neonatal intensive care units worldwide, causing significant morbidity and increased health care costs. The increasing antibiotic resistance and the ability to form biofilms are important determinants of pathogenicity of CNS infections. Several strategies have been developed to prevent CoNS LOS. These include methods to prevent biofilm formation by blocking molecular mechanisms of biofilm formation or impregnating catheters with antimicrobial agents and immune enhancement strategies. Most of these strategies are still under development. However, interventions such as promotion of hygiene and application of catheter lock techniques have been shown to decrease CoNS LOS. In this review, we summarize identified risk factors, both bacterial and host related, for CoNS LOS and discuss preventive strategies.
Prevention of CoNS LOS in neonates 111
Ch
ap
ter
6
Introduction
Bacterial sepsis is an important cause of morbidity and mortality among neonates at neonatal intensive care units (NICU) (Figure 1) (1, 2). Traditionally, neonatal sepsis can be divided into two categories, based on their time of onset. Early-onset sepsis is defined as sepsis occurring within the first 48-72 hours after birth, while late-onset sepsis (LOS) occurs after 48-72 hours after birth. Infections leading to early-onset sepsis are acquired perinatally, with Escherichia coli and Group B Streptococci as the major causative agents (3). Late-onset sepsis, on the other hand, is usually a nosocomial infection caused by a Gram-positive microorganism. Coagulase-negative staphylococci (CoNS) are the most frequently isolated pathogens in LOS, with incidence rates up to 75-80%. CoNS LOS is associated with low birth weight (BW) and low gestational age (GA) (4, 5). Although CoNS LOS does not cause much mortality, it does cause high morbidity leading to prolonged hospitalization and thus an increased healthcare cost of more than $ 25,000.- per infant suffering from CoNS LOS (6). Since antibiotic resistance in CoNS has dramatically increased (7-9), prevention of infections by CoNS LOS is probably the most effective way to decrease morbidity by these organisms. In this review, we summarize known risk factors, both bacterial and host related, for CoNS LOS and discuss preventive strategies. Preventive strategies that have already shown clinical effect and can easily be implemented in NICUs are shown in Table 1.
Figure 1. Infant at the NICU.
Table 1. Implementable CoNS LOS prevention strategies
Prevention strategy References
Strict adherence to hand hygiene measures on the NICU 77-79
Usage of a vancomycin-heparin lock (0.4 mL of heparinized saline with 25μg/mL vancomycin, twice daily for 20-60 minutes) 51
Aseptic insertion and management of central venous catheters 86-87
Administration of cefazolin 100 mg/kg/day in two doses, 1 hour before and 12 hours after removal of a central vascular catheter 89
Avoidance of the use of topical ointments 90
Early start of small amounts of enteral feeding in neonates 93
Lower extremity insertion of central venous catheters for TPN 95
Chapter 6112
CoNS and LOSThe group of coagulase-negative staphylococci consists of almost 40 known species and subspecies (10). Approximately half of these can be found in humans. As mentioned before, CoNS are commensal bacteria, which belong to the normal flora of skin and mucosa. Treatment of CoNS sepsis is usually equal for all CoNS species. As a consequence the majority of studies on neonatal sepsis do not specify the isolated CoNS species. Studies in which CoNS species identification has been performed show that only a small number of CoNS species cause LOS. The largest group within the species is Staphylococcus epidermidis, accounting for 58-90% of isolated CoNS. Other species include Staphylococcus haemolyticus, Staphylococcus capitis, Staphylococcus hominis and Staphylococcus warneri (9, 11). Not surprisingly, S. epidermidis is the best studied CoNS species and much is known about its virulence factors like biofilm formation. S. epidermidis is therefore often used as the model species in CoNS research. Since CoNS are commensal bacteria, there is frequently debate whether they are true pathogens or not. As early as in 1987 Schmidt et. al. already showed that CoNS blood culture positive LOS was no different from LOS by other pathogens regarding clinical and laboratory outcomes (12). Others proposed that many of the CoNS positive blood cultures might be contaminants (13). Therefore, most studies use strict definitions for CoNS sepsis, such as no other species in the blood culture, a CoNS positive blood culture with laboratory signs of infection, raised CRP, or a second CoNS positive blood culture from a different site from the patient (1, 14). The United Stated Centers for Disease Control and Prevention (CDC) definition of primary bloodsteam infection states that the patient has either fever, hypothermia, apnea and/or bradycardia AND two positive blood cultures drawn on separate occasions with an organism that is not related to infection at another site or a positive blood culture from a patient with an intravascular access device with the physician treating the patient with appropriate antibiotics (15).
Bacterial risk factors and preventive measures
Antibiotic resistanceAntibiotic resistance itself is not a virulence factor. However, CoNS isolated from patients with LOS are mostly multiresistant and/or mecA positive, in contrast to community-acquired CoNS (8, 9, 11, 14, 16). Since antibiotics are extensively used at NICUs, antibiotic resistance is probably an important selective force and not a prerequisite for infection. Antibiotic resistance is associated with biofilm formation, the most important virulence factor of CoNS, causing complications in eradication strategies (11, 14). A potential danger of the abundant presence of mecA positive S. epidermidis is the possibility to horizontally transfer mecA DNA to Staphylococcus aureus, thus creating methicillin resistant S. aureus (17). It is therefore important to be restrictive with antibiotics to prevent resistance.
Vancomycin prophylaxis As a large portion infecting CoNS have proven to be multiresistant, especially to β-lactam antibiotics, vancomycin is the preferred antibiotic for the treatment of CoNS LOS in most hospitals. The use of vancomycin as a prophylaxis to prevent CoNS LOS has been studied both as continuous infusion of 25 μg/ml in hyperalimentation, as well as a twice daily regimen of 5mg/kg (18-21). All studies showed a decrease in CoNS sepsis when receiving
Prevention of CoNS LOS in neonates 113
Ch
ap
ter
6
prophylactic vancomycin. A meta-analysis of five studies showed a decrease in incidence of sepsis by all pathogens as well as by CoNS sepsis (RR 0.11, 95% CI 0.05, 0.24 and RR 0.33, 95% CI 0.19, 0.59 respectively). Continuous infusion of vancomycin proved to be more effective than intermittent treatment. Despite these results, there was no significant difference in hospital mortality, length of NICU stay and toxic effects of vancomycin between the prophylaxis and non-prophylaxis groups (22). No randomized controlled trials have yet been performed to study the effect of prophylaxis on vancomycin resistance. However, vancomycin resistance in coagulase-negative staphylococci has been described in several cases. Therefore, vancomycin profylaxis is not advised (23-27).
BiofilmsBiofilm formation is the most important virulence factor of CoNS in LOS. Bacteria in a biofilm are inherently resistant to host immune responses and antibiotics (28). Since many neonates have intravascular catheters for nutrition and medication, CoNS can grow biofilms on them and subsequently spread into the bloodstream. Intravascular access is limited in premature infants and thus removal of the catheter is not always possible. Attacking biofilms or biofilm formation would therefore be effective prevention for CoNS LOS. Thorough understanding of the process of biofilm formation is necessary to develop effective strategies against biofilms. Several excellent reviews have been published on the mechanisms of biofilm formation in S. epidermidis (29-31). In short, biofilm formation consists of three phases. Initially, the bacteria attach to the intravascular catheters using aspecific factors like Van Der Waal’s forces and hydrophobic interactions. Secondly, attachment to extracellular matrix host proteins, which have coated the catheter, occurs. In this phase, surface-exposed proteins like the fibrinogen-binding protein (Fbe) and autolysin E (AtlE) play a major role. In the third phase, bacteria accumulate and proliferate. In this phase, the accumulation associated protein (Aap) and the production of polysaccharide intercellular adhesion (PIA) are thought to play an important role (Figure 2). Although this process describes biofilm formation in S. epidermidis, other CoNS appear to use the same basic mechanisms (32). Knowledge of this process has encouraged the development of several strategies against CoNS biofilms.
Figure 2. Biofilm formation on an intravascular catheter. A) CoNS on the skin attach to the catheter by aspecific factors like hydrophobic interactions. B) Inside the vessel, the catheter is coated by host matrix proteins like fibrin and fibrinogen. Using surface proteins like Fbe and AtlE, CoNS attach to these host proteins. C) Polysaccharide, specific proteins like AAP and accessory macromolecules provide intercellular aggregation and bacteria proliferate and accumulate in multilayered cell clusters. D) The biofilm matures and detaches. Mechanism are poorly understood, but quorum sensing controlled expression of detergent-like peptides and proteolytic activity is might be involved.
Chapter 6114
Prevention of biofilm formation One strategy to prevent biofilm formation is to interrupt the mechanism of biofilm formation. Much research has been done on the structure and function of PIA. PIA is coded by the icaADBC operon and it has been found that invasive S. epidermidis strains were more often icaADBC positive than commensal S. epidermidis strains (33-35). Several studies were conducted on the inhibition of PIA. Antibacterial agents like povidone-iodine and hydrogen peroxide, but also nitrite decrease biofilm formation of S. epidermidis by a diminished transcription of icaADBC (36-38). Recently, a Spanish group has shown that allicin inhibits biofilm formation in S. epidermidis, probably by a specific enzymatic inhibition in PIA synthesis (39). Other studies have focused on the prevention of biofilm formation by blocking key molecules associated with biofilm formation. In 2001, Pei et al. have shown that antibodies against Fbe block adherence of S. epidermidis to catheters (40). Antibodies against other surface-exposed proteins like Aap and the type I collagen binding SdrF have also shown to inhibit biofilm formation or reduce attachment to catheters (41, 42). The publication of the S. epidermidis genome has opened the possibility to perform computer based analyses on the genome to identify unknown surface-exposed proteins and study them as possible targets for biofilm inhibition (43-45). Recently, Shahrooei et al. discovered that SesC, a S. epidermidis surface-exposed protein of yet unknown function, plays a role in both primary attachment and biofilm accumulation phases and that antibodies against SesC inhibit biofilm formation (46). Although none of these biofilm inhibiting agents have been tested clinically, the prospects are quite promising. Unfortunately, these experiments all focus on S. epidermidis and molecular mechanisms of biolfilm formation in other CoNS may vary. For example, recently Fredheim et al. have shown that PIA only plays a minor role in biofilm formation of S. haemolyticus (47). As outbreaks of other CoNS then S. epidermidis have been described (23, 48, 49), studies of biofilm formation should not be limited to S. epidermidis, but extended to all CoNS. Using the knowledge of the biofilm mechanisms of all CoNS species, the most effective and efficient prevention strategies can be designed.
Catheter lock solutions Another well studied strategy to prevent catheter colonization is the catheter lock technique. In this technique, the lumen of the catheter is filled with a high concentration (100-1000 times higher than in systemic application) of an antibiotic while the catheter is not in use (50). Numerous studies, comparing the effects of several antimicrobial agents have been published. In a randomized controlled trial, Garland et al. showed that the use of a vancomycin-heparin lock (0.4 mL of heparinized saline with 25μg/mL vancomycin, twice daily for 20-60 minutes) reduced the incidence of catheter related blood stream infections in neonates (RR 0.16, 95% CI 0.04 – 0.66), compared to a catheter lock with just heparinized saline. Vancomycin resistant Gram-positive bacteria were not isolated from these infants (51). More recently, Qu et al. have shown that for the eradication of coagulase-negative staphylococcal biofilms in vitro, a low concentration ethanol lock is more effective than a lock with conventional antibiotics (52). Chambers et al. have shown the effectiveness of ethanol locks in sheep in a double blind randomized trial (53). Since ethanol locks may even be more beneficial than vancomycin locks, prospective randomized controlled trials should be conducted in the NICU.
Prevention of CoNS LOS in neonates 115
Ch
ap
ter
6
Other catheter-mediated solutionsThere are many methods to reduce catheter related infection by coating or impregnating of catheters. These include fish protein coating, impregnation with chlorhexidine, silver sulfadiazine, cobalt, copper, antibiotics, phages and incorporation of phages in hydrogel coating of catheters (54-58). Rabinovitch and Stewart have shown that electrolysis can remove and inactivate S. epidermidis biofilms (59). Unfortunately, impregnation and other catheter related antimicrobial solutions are either experimental or tested in adults and thus not yet an option in neonates (60).
Host risk factors and prevention measures
Impaired immune systemLow birth weight and low gestational age are important risk factors for LOS and in particular CoNS LOS, as decreasing birth weight and gestational age increases risk for LOS (1, 4, 5). This association is mainly due to a relatively immature immune system in small and premature infants (61). The opsonic activity against S. epidermidis and the level of IgG antibodies to staphylococcal peptidoglycan in serum from premature newborns correlate with gestational age (62). Furthermore, complement activity of both classic and alternative pathways is significantly reduced in preterm infants (63). Also, Björkvist et al. found that the neutrophil oxidative burst on exposure to CoNS was defective in premature infants (64).
Immune therapySeveral attempts have been made to reduce CoNS LOS by enhancement of the neonatal immune system. Prophylactic administration of intravenous immune globulins (IVIG) to prematures only showed a slight non-significant reduction in the incidence of CoNS LOS (65, 66). Antibodies against CoNS lipoteichoic acid, PIA and Fbe have opsonophagocytic properties in vitro, making these antigens promising targets for monoclonal antibodies (67-70). Studies using several antistaphylococcal immunoglobulins have been performed, most without success (71). A recent phase 2, randomized, double-blind, placebo-controlled study on Pagibaximab, a human chimeric monoclonal antibody developed against lipoteichoic acid showed that this antibody seemed safe and well tolerated. Although differences in staphylococcal sepsis between study and placebo groups were not significant, no staphylococcal sepsis occured in the group that had received the highest dose (72). The disappointing results of prophylactic antibody administration suggest that other immune deficiencies might be more important in CoNS LOS. A better understanding of the neonatal immune system, and more specifically it’s interaction with CoNS, is necessary for the development of immune enhancing strategies.
Hygiene As clonality among LOS isolated CoNS strains is common, the lack of hand hygiene is thought to be an important cause for the spread of infecting CoNS (5, 8, 9, 11, 73-75). It has been shown that CoNS isolates from LOS can be found on hands of NICU personnel (16, 76). NICU personnel is colonized with multiresistant strains when working on the NICU, but not after a period of absence (16). Colonization of neonates with antimicrobial resistant CoNS increases during hospitalization on the NICU (77). Therefore strict hygiene measurements within the NICU are of absolute importance. It has been demonstrated that an evidence-
Chapter 6116
based hand washing policy results in a significant decrease in false-positive CoNS blood culture rates and possibly also true-positive CoNS blood culture rates (78). A study on the impact of standardized hand hygiene programs showed a reduction from 19% to 6% in nosocomial infections after implementation of the program (79). However, as compliance to hand hygiene decreases over time, it is necessary to regularly educate NICU personnel on the correct procedures and usefulness of hand hygiene measurements (80).
Intravascular catheters The presence of intravascular catheters is an important risk factor for CoNS LOS, since they can grow biofilms on them (5, 81-84). The most effective method for treating recurrent CoNS sepsis is to simply remove the catheter and thus remove the source of the bacteremia (85, 86). However, this is not always possible as intravenous access is limited in neonates and catheters are necessary to administer nutrition and medication and to perform blood gas analyses. In general, it has been shown that maximal sterile barrier precautions for insertion of central venous catheters, like the use of sterile gowns, gloves, caps and masks, minimized infections (87). Aly et al. managed to drastically reduce nosocomial blood stream infections in a NICU by changing several practices, like the use of a closed medication system and using sterile techniques (88). Van den Hoogen et al. suggested that the removal of percutaneously inserted central venous catheters from neonates is in itself a risk factor for sepsis (89). CoNS sepsis associated with catheter removal could be prevented by administration of cefazolin 100 mg/kg/day in two doses, 1 hour before and 12 hours after removal of the catheter (90). As catheters disrupt the already immature and fragile skin of neonates, topical ointments were proposed as enhancement of the epidermal barrier function of the skin. A Cochrane review by Conner et al. showed that the use of topical ointments increases the risk of CoNS sepsis and any nosocomial infection in neonates, rather than decreasing it (91). Topical ointments should therefore be avoided.
Total parenteral nutritionThe intravenous administration of lipids with total parenteral nutrition (TPN) is another well documented risk factor for CoNS LOS (81-84). A possible reason is that TPN is administered to the smallest and sickest infants, who are already at high risk. However, large concentrations of lipids are a great growth media for CoNS (83). Also, infant neutrophil phagocytosis and the killing of CoNS as well as CoNS-induced tumour necrosis factor-α (TNF-α) in vitro is impaired by TPN (92, 93). Small volumes of enteral feedings were shown to normalize neonatal immune function in vitro, possibly due to an immune modulation by stimulation of the gastrointestinal tract (94). Whereas it is generally advised to insert central catheters in the upper extremity (95), Hoang et al. showed that for the administration of TPN in neonates, lower extremity inserted catheters had lower rates of CoNS LOS compared to upper extremity inserted catheters (50% vs 86%, P<0.05) (96).
Summary and recommendations
CoNS remain the most important pathogen in LOS on NICUs worldwide, causing significant morbidity and increased health care costs. Many intervention strategies to prevent CoNS
Prevention of CoNS LOS in neonates 117
Ch
ap
ter
6
LOS are being studied. Proper hand hygiene is probably the most effective measure, but compliance to hand hygiene measurements decreases over time. Several other strategies, like the development of biofilm blocking measurements are promising, but need more research to ensure clinical usefulness. Methods to promote immune enhancement have been less successful. Therefore and in view of the significant costs of monoclonal antibodies, the clinical applications of methods to promote immune enhancement with antibodies may be restricted. To decrease the incidence of CoNS LOS, implementation of the strategies in Table 1 should be considered, although randomized controlled trials need to be performed to prove the true effectiveness of these strategies.
Chapter 6118
References
1. Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285-291.
2. Stoll BJ, Hansen N, Fanaroff AA, et al. Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants. N Engl J Med. 2002;347:240-247.
3. Stoll BJ, Hansen NI, Higgins RD, et al. Very low birth weight preterm infants with early onset neonatal sepsis: the predominance of gram-negative infections continues in the National Institute of Child Health and Human Development Neonatal Research Network, 2002-2003. Pediatr Infect Dis J. 2005;24:635-639.
4. Healy CM, Palazzi DL, Edwards MS, Campbell JR, Baker CJ. Features of invasive staphylococcal disease in neonates. Pediatrics. 2004;114:953-961.
5. Vermont CL, Hartwig NG, Fleer A, et al. Persistence of clones of coagulase-negative staphylococci among premature neonates in neonatal intensive care units: two-center study of bacterial genotyping and patient risk factors. J Clin Microbiol. 1998;36:2485-2490.
6. Gray JE, Richardson DK, McCormick MC, Goldmann DA. Coagulase-negative staphylococcal bacteremia among very low birth weight infants: relation to admission illness severity, resource use, and outcome. Pediatrics. 1995;95:225-230.
7. De Giusti M, Pacifico L, Tufi D, et al. Phenotypic detection of nosocomial mecA-positive coagulase-negative staphylococci from neonates. J Antimicrob Chemother. 1999;44:351-358.
8. Krediet TG, Mascini EM, van Rooij E, et al. Molecular epidemiology of coagulase-negative staphylococci causing sepsis in a neonatal intensive care unit over an 11-year period. J Clin Microbiol. 2004;42:992-995.
9. Raimundo O, Heussler H, Bruhn JB, et al. Molecular epidemiology of coagulase-negative staphylococcal bacteraemia in a newborn intensive care unit. J Hosp Infect. 2002;51:33-42.
10. Trulzsch K, Grabein B, Schumann P, et al. Staphylococcus pettenkoferi sp. nov., a novel coagulase-negative staphylococcal species isolated from human clinical specimens. Int J Syst Evol Microbiol. 2007;57:1543-1548.
11. Hira V, Sluijter M, Estevao S, et al. Clinical and molecular epidemiologic characteristics of coagulase-negative staphylococcal bloodstream infections in intensive care neonates. Pediatr Infect Dis J. 2007;26:607-612.
12. Schmidt BK, Kirpalani HM, Corey M, et al. Coagulase-negative staphylococci as true pathogens in newborn infants: a cohort study. Pediatr Infect Dis J. 1987;6:1026-1031.
13. Calnen G, Campognone P, Peter G. Coagulase-negative staphylococcal bacteremia in newborns. Clin Pediatr (Phila). 1984;23:542-544.
14. Klingenberg C, Aarag E, Ronnestad A, et al. Coagulase-negative staphylococcal sepsis in neonates. Association between antibiotic resistance, biofilm formation and the host inflammatory response. Pediatr Infect Dis J. 2005;24:817-822.
15. Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128-140.
16. Hira V, Sluijter M, Goessens WH, et al. Coagulase-negative staphylococcal skin carriage among neonatal intensive care unit personnel: from population to infection. J Clin Microbiol. 2010;48:3876-3881.
17. Wielders CL, Vriens MR, Brisse S, et al. In-vivo transfer of mecA DNA to Staphylococcus aureus [corrected]. Lancet. 2001;357:1674-1675.
18. Baier RJ, Bocchini JA, Jr., Brown EG. Selective use of vancomycin to prevent coagulase-negative staphylococcal nosocomial bacteremia in high risk very low birth weight infants. Pediatr Infect Dis J. 1998;17:179-183.
19. Cooke RW, Nycyk JA, Okuonghuae H, et al. Low-dose vancomycin prophylaxis reduces coagulase-negative staphylococcal bacteraemia in very low birthweight infants. J Hosp Infect. 1997;37:297-303.
20. Kacica MA, Horgan MJ, Ochoa L, et al. Prevention of gram-positive sepsis in neonates weighing less than 1500 grams. J Pediatr. 1994;125:253-258.
21. Spafford PS, Sinkin RA, Cox C, Reubens L, Powell KR. Prevention of central venous catheter-related coagulase-negative staphylococcal sepsis in neonates. J Pediatr. 1994;125:259-263.
22. Craft AP, Finer NN, Barrington KJ. Vancomycin for prophylaxis against sepsis in preterm neonates. Cochrane Database Syst Rev. 2000:CD001971.
23. Center KJ, Reboli AC, Hubler R, Rodgers GL, Long SS. Decreased vancomycin susceptibility of coagulase-negative staphylococci in a neonatal intensive care unit: evidence of spread of Staphylococcus warneri. J Clin Microbiol. 2003;41:4660-4665.
24. Krcmery V, Jr., Trupl J, Drgona L, et al. Nosocomial bacteremia due to vancomycin-resistant Staphylococcus epidermidis in four patients with cancer, neutropenia, and previous treatment with vancomycin. Eur J Clin Microbiol Infect Dis. 1996;15:259-261.
25. Palazzo IC, Araujo ML, Darini AL. First report of vancomycin-resistant staphylococci isolated from healthy carriers in Brazil. J Clin Microbiol. 2005;43:179-185.
26. Sanyal D, Johnson AP, George RC, Cookson BD, Williams AJ. Peritonitis due to vancomycin-resistant Staphylococcus epidermidis. Lancet. 1991;337:54.
Prevention of CoNS LOS in neonates 119
Ch
ap
ter
6
27. Guidelines for the prevention of intravascular related infections 2002. MMWR Recommendations and Reports: Center for Disease Control; 2002:10.
28. Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318-1322.
29. Otto M. Staphylococcus epidermidis--the ‘accidental’ pathogen. Nat Rev Microbiol. 2009;7:555-567.30. Rohde H, Frankenberger S, Zahringer U, Mack D. Structure, function and contribution of polysaccharide
intercellular adhesin (PIA) to Staphylococcus epidermidis biofilm formation and pathogenesis of biomaterial-associated infections. Eur J Cell Biol. 2010;89:103-111.
31. von Eiff C, Peters G, Heilmann C. Pathogenesis of infections due to coagulase-negative staphylococci. Lancet Infect Dis. 2002;2:677-685.
32. Otto M. Staphylococcal biofilms. Curr Top Microbiol Immunol. 2008;322:207-228.33. Frebourg NB, Lefebvre S, Baert S, Lemeland JF. PCR-Based assay for discrimination between invasive and
contaminating Staphylococcus epidermidis strains. J Clin Microbiol. 2000;38:877-880.34. Li H, Xu L, Wang J, et al. Conversion of Staphylococcus epidermidis strains from commensal to invasive by
expression of the ica locus encoding production of biofilm exopolysaccharide. Infect Immun. 2005;73:3188-3191.
35. Mack D, Haeder M, Siemssen N, Laufs R. Association of biofilm production of coagulase-negative staphylococci with expression of a specific polysaccharide intercellular adhesin. J Infect Dis. 1996;174:881-884.
36. Glynn AA, O’Donnell ST, Molony DC, et al. Hydrogen peroxide induced repression of icaADBC transcription and biofilm development in Staphylococcus epidermidis. J Orthop Res. 2009;27:627-630.
37. Oduwole KO, Glynn AA, Molony DC, et al. Anti-biofilm activity of sub-inhibitory povidone-iodine concentrations against Staphylococcus epidermidis and Staphylococcus aureus. J Orthop Res. 2010;28:1252-1256.
38. Schlag S, Nerz C, Birkenstock TA, Altenberend F, Gotz F. Inhibition of staphylococcal biofilm formation by nitrite. J Bacteriol. 2007;189:7911-7919.
39. Cruz-Villalon G, Perez-Giraldo C. Effect of allicin on the production of polysaccharide intercellular adhesin in Staphylococcus epidermidis. J Appl Microbiol. 2010.
40. Pei L, Flock JI. Functional study of antibodies against a fibrogenin-binding protein in Staphylococcus epidermidis adherence to polyethylene catheters. J Infect Dis. 2001;184:52-55.
41. Arrecubieta C, Toba FA, von Bayern M, et al. SdrF, a Staphylococcus epidermidis surface protein, contributes to the initiation of ventricular assist device driveline-related infections. PLoS Pathog. 2009;5:e1000411.
42. Sun D, Accavitti MA, Bryers JD. Inhibition of biofilm formation by monoclonal antibodies against Staphylococcus epidermidis RP62A accumulation-associated protein. Clin Diagn Lab Immunol. 2005;12:93-100.
43. Bowden MG, Chen W, Singvall J, et al. Identification and preliminary characterization of cell-wall-anchored proteins of Staphylococcus epidermidis. Microbiology. 2005;151:1453-1464.
44. Gill SR, Fouts DE, Archer GL, et al. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J Bacteriol. 2005;187:2426-2438.
45. Zhang YQ, Ren SX, Li HL, et al. Genome-based analysis of virulence genes in a non-biofilm-forming Staphylococcus epidermidis strain (ATCC 12228). Mol Microbiol. 2003;49:1577-1593.
46. Shahrooei M, Hira V, Stijlemans B, et al. Inhibition of Staphylococcus epidermidis biofilm formation by rabbit polyclonal antibodies against the SesC protein. Infect Immun. 2009;77:3670-3678.
47. Fredheim EG, Klingenberg C, Rohde H, et al. Biofilm formation by Staphylococcus haemolyticus. J Clin Microbiol. 2009;47:1172-1180.
48. Neumeister B, Kastner S, Conrad S, Klotz G, Bartmann P. Characterization of coagulase-negative staphylococci causing nosocomial infections in preterm infants. Eur J Clin Microbiol Infect Dis. 1995;14:856-863.
49. Van Der Zwet WC, Debets-Ossenkopp YJ, Reinders E, et al. Nosocomial spread of a Staphylococcus capitis strain with heteroresistance to vancomycin in a neonatal intensive care unit. J Clin Microbiol. 2002;40:2520-2525.
50. Messing B, Peitra-Cohen S, Debure A, Beliah M, Bernier JJ. Antibiotic-lock technique: a new approach to optimal therapy for catheter-related sepsis in home-parenteral nutrition patients. JPEN J Parenter Enteral Nutr. 1988;12:185-189.
51. Garland JS, Alex CP, Henrickson KJ, McAuliffe TL, Maki DG. A vancomycin-heparin lock solution for prevention of nosocomial bloodstream infection in critically ill neonates with peripherally inserted central venous catheters: a prospective, randomized trial. Pediatrics. 2005;116:e198-205.
52. Qu Y, Istivan TS, Daley AJ, Rouch DA, Deighton MA. Comparison of various antimicrobial agents as catheter lock solutions: preference for ethanol in eradication of coagulase-negative staphylococcal biofilms. J Med Microbiol. 2009;58:442-450.
53. Chambers ST, Pithie A, Gallagher K, et al. Treatment of Staphylococcus epidermidis central vascular catheter infection with 70% ethanol locks: efficacy in a sheep model. J Antimicrob Chemother. 2007;59:779-782.
54. Lu TK, Collins JJ. Dispersing biofilms with engineered enzymatic bacteriophage. Proc Natl Acad Sci U S A. 2007;104:11197-11202.
Chapter 6120
55. Maki DG, Stolz SM, Wheeler S, Mermel LA. Prevention of central venous catheter-related bloodstream infection by use of an antiseptic-impregnated catheter. A randomized, controlled trial. Ann Intern Med. 1997;127:257-266.
56. Raad I, Darouiche R, Dupuis J, et al. Central venous catheters coated with minocycline and rifampin for the prevention of catheter-related colonization and bloodstream infections. A randomized, double-blind trial. The Texas Medical Center Catheter Study Group. Ann Intern Med. 1997;127:267-274.
57. Vejborg RM, Klemm P. Blocking of bacterial biofilm formation by a fish protein coating. Appl Environ Microbiol. 2008;74:3551-3558.
58. Wood P, Jones M, Bhakoo M, Gilbert P. A Novel Strategy for Control of Microbial Biofilms through Generation of Biocide at the Biofilm-Surface Interface. Appl Environ Microbiol. 1996;62:2598-2602.
59. Rabinovitch C, Stewart PS. Removal and inactivation of Staphylococcus epidermidis biofilms by electrolysis. Appl Environ Microbiol. 2006;72:6364-6366.
60. Gilbert RE, Harden M. Effectiveness of impregnated central venous catheters for catheter related blood stream infection: a systematic review. Curr Opin Infect Dis. 2008;21:235-245.
61. Strunk T, Richmond P, Simmer K, et al. Neonatal immune responses to coagulase-negative staphylococci. Curr Opin Infect Dis. 2007;20:370-375.
62. Fleer A, Gerards LJ, Aerts P, et al. Opsonic defense to Staphylococcus epidermidis in the premature neonate. J Infect Dis. 1985;152:930-937.
63. Notarangelo LD, Chirico G, Chiara A, et al. Activity of classical and alternative pathways of complement in preterm and small for gestational age infants. Pediatr Res. 1984;18:281-285.
64. Bjorkqvist M, Jurstrand M, Bodin L, Fredlund H, Schollin J. Defective neutrophil oxidative burst in preterm newborns on exposure to coagulase-negative staphylococci. Pediatr Res. 2004;55:966-971.
65. Baker CJ, Melish ME, Hall RT, et al. Intravenous immune globulin for the prevention of nosocomial infection in low-birth-weight neonates. The Multicenter Group for the Study of Immune Globulin in Neonates. N Engl J Med. 1992;327:213-219.
66. Weisman LE, Stoll BJ, Kueser TJ, et al. Intravenous immune globulin prophylaxis of late-onset sepsis in premature neonates. J Pediatr. 1994;125:922-930.
67. Maira-Litran T, Kropec A, Abeygunawardana C, et al. Immunochemical properties of the staphylococcal poly-N-acetylglucosamine surface polysaccharide. Infect Immun. 2002;70:4433-4440.
68. Rennermalm A, Nilsson M, Flock JI. The fibrinogen binding protein of Staphylococcus epidermidis is a target for opsonic antibodies. Infect Immun. 2004;72:3081-3083.
69. Vernachio JH, Bayer AS, Ames B, et al. Human immunoglobulin G recognizing fibrinogen-binding surface proteins is protective against both Staphylococcus aureus and Staphylococcus epidermidis infections in vivo. Antimicrob Agents Chemother. 2006;50:511-518.
70. Weisman LE. Coagulase-negative staphylococcal disease: emerging therapies for the neonatal and pediatric patient. Curr Opin Infect Dis. 2004;17:237-241.
71. Shah PS, Kaufman DA. Antistaphylococcal immunoglobulins to prevent staphylococcal infection in very low birth weight infants. Cochrane Database Syst Rev. 2009:CD006449.
72. Weisman LE, Thackray HM, Steinhorn RH, et al. A randomized study of a monoclonal antibody (pagibaximab) to prevent staphylococcal sepsis. Pediatrics. 2011;128:271-279.
73. Boisson K, Thouverez M, Talon D, Bertrand X. Characterisation of coagulase-negative staphylococci isolated from blood infections: incidence, susceptibility to glycopeptides, and molecular epidemiology. Eur J Clin Microbiol Infect Dis. 2002;21:660-665.
74. Monsen T, Karlsson C, Wistrom J. Spread of clones of multidrug-resistant, coagulase-negative staphylococci within a university hospital. Infect Control Hosp Epidemiol. 2005;26:76-80.
75. Villari P, Sarnataro C, Iacuzio L. Molecular epidemiology of Staphylococcus epidermidis in a neonatal intensive care unit over a three-year period. J Clin Microbiol. 2000;38:1740-1746.
76. Patrick CH, John JF, Levkoff AH, Atkins LM. Relatedness of strains of methicillin-resistant coagulase-negative Staphylococcus colonizing hospital personnel and producing bacteremias in a neonatal intensive care unit. Pediatr Infect Dis J. 1992;11:935-940.
77. Hira V, Kornelisse RF, Sluijter M, et al. Colonization dynamics of antibiotic-resistant coagulase-negative staphylococci in neonates. J Clin Microbiol. 2013;51:595-597.
78. Sharek PJ, Benitz WE, Abel NJ, et al. Effect of an evidence-based hand washing policy on hand washing rates and false-positive coagulase negative staphylococcus blood and cerebrospinal fluid culture rates in a level III NICU. J Perinatol. 2002;22:137-143.
79. Capretti MG, Sandri F, Tridapalli E, et al. Impact of a standardized hand hygiene program on the incidence of nosocomial infection in very low birth weight infants. Am J Infect Control. 2008;36:430-435.
80. Allegranzi B, Pittet D. Role of hand hygiene in healthcare-associated infection prevention. J Hosp Infect. 2009;73:305-315.
Prevention of CoNS LOS in neonates 121
Ch
ap
ter
6
81. Anderson-Berry A, Brinton B, Lyden E, Faix RG. Risk factors associated with development of persistent coagulase-negative staphylococci bacteremia in the neonate and associated short-term and discharge morbidities. Neonatology. 2011;99:23-31.
82. Avila-Figueroa C, Goldmann DA, Richardson DK, et al. Intravenous lipid emulsions are the major determinant of coagulase-negative staphylococcal bacteremia in very low birth weight newborns. Pediatr Infect Dis J. 1998;17:10-17.
83. Freeman J, Goldmann DA, Smith NE, et al. Association of intravenous lipid emulsion and coagulase-negative staphylococcal bacteremia in neonatal intensive care units. N Engl J Med. 1990;323:301-308.
84. Nataro JP, Corcoran L, Zirin S, et al. Prospective analysis of coagulase-negative staphylococcal infection in hospitalized infants. J Pediatr. 1994;125:798-804.
85. Karlowicz MG, Furigay PJ, Croitoru DP, Buescher ES. Central venous catheter removal versus in situ treatment in neonates with coagulase-negative staphylococcal bacteremia. Pediatr Infect Dis J. 2002;21:22-27.
86. Raad I, Kassar R, Ghannam D, et al. Management of the catheter in documented catheter-related coagulase-negative staphylococcal bacteremia: remove or retain? Clin Infect Dis. 2009;49:1187-1194.
87. Walz JM, Memtsoudis SG, Heard SO. Analytic reviews: prevention of central venous catheter bloodstream infections. J Intensive Care Med. 2010;25:131-138.
88. Aly H, Herson V, Duncan A, et al. Is bloodstream infection preventable among premature infants? A tale of two cities. Pediatrics. 2005;115:1513-1518.
89. van den Hoogen A, Brouwer MJ, Gerards LJ, Fleer A, Krediet TG. Removal of percutaneously inserted central venous catheters in neonates is associated with the occurrence of sepsis. Acta Paediatr. 2008;97:1250-1252.
90. Hemels MA, van den Hoogen A, Verboon-Maciolek MA, Fleer A, Krediet TG. Prevention of neonatal late-onset sepsis associated with the removal of percutaneously inserted central venous catheters in preterm infants. Pediatr Crit Care Med. 2011.
91. Conner JM, Soll RF, Edwards WH. Topical ointment for preventing infection in preterm infants. Cochrane Database Syst Rev. 2004:CD001150.
92. Okada Y, Klein NJ, van Saene HK, et al. Bactericidal activity against coagulase-negative staphylococci is impaired in infants receiving long-term parenteral nutrition. Ann Surg. 2000;231:276-281.
93. Okada Y, Papp E, Klein NJ, Pierro A. Total parenteral nutrition directly impairs cytokine production after bacterial challenge. J Pediatr Surg. 1999;34:277-280.
94. Okada Y, Klein N, van Saene HK, Pierro A. Small volumes of enteral feedings normalise immune function in infants receiving parenteral nutrition. J Pediatr Surg. 1998;33:16-19.
95. O’Grady NP, Alexander M, Dellinger EP, et al. Guidelines for the prevention of intravascular catheter-related infections. The Hospital Infection Control Practices Advisory Committee, Center for Disease Control and Prevention, U.S. Pediatrics. 2002;110:e51.
96. Hoang V, Sills J, Chandler M, et al. Percutaneously inserted central catheter for total parenteral nutrition in neonates: complications rates related to upper versus lower extremity insertion. Pediatrics. 2008;121:e1152-1159.
Chapter
7
Summarizing discussionFuture perspectives
Nederlandse samenvattingToekomstperspectieven
122
Chapter 7124
Summarizing discussion
Coagulase-negative staphylococci (CoNS) are the leading cause of late-onset sepsis (LOS) worldwide (1). Although mortality by CoNS LOS is low, the high morbidity leads to prolonged hospitalization and thus increased healthcare costs (2). As antibiotic resistance among CoNS is dramatically increasing (3-5), preventive measures become more and more important.
In chapter 2 we described the incidence of CoNS LOS in a large NICU and identified patient characteristics associated with LOS. Furthermore, the use of restriction fragment end labelling (RFEL) as a tool for epidemiological molecular typing of CoNS was evaluated in this chapter. We observed that CoNS were isolated in 70% of the bloodstream infections among VLBW infants. As a hospitalization of more than 14 days was significantly associated with CoNS LOS (odds ratio 6.0, median age at first infection 8 days), CoNS LOS is clearly a problem on the NICU. S. epidermidis and S. haemolyticus were shown to be the most prevalent species with 32% of all isolates belonging to one single genetic cluster. This finding was comparable with other studies, suggesting that cross-contamination, for example due to insufficient hand hygiene, may be an important factor in CoNS infection (4, 6, 7). Furthermore, we showed that less than half of the isolates displayed the in vitro biofilm producing phenotype, whereas antibiotic resistance was abundant in the isolated strains. PCR on the mecA gene was positive in 87%, 77% was multidrug-resistant. Pulsed-field gel electrophoresis (PFGE) is the most commonly used method for molecular typing of CoNS (4, 5, 7, 8). We validated restriction fragment end labelling (RFEL) as an alternative tool for molecular typing of CoNS by identifying the concordance between RFEL and PFGE and found that these two techniques matched quite well with a significant r value of 74%.
In chapter 3 we studied the carriage of CoNS on the hands of NICU personnel. It had already been shown that carriage of antibiotic-resistant CoNS among hospital personnel increases during employment (9, 10). We have shown that carriage of antibiotic-resistant CoNS also decreases after a period of absence from the hospital environment. Together with the striking finding that 90% of the blood isolates could be found on the hands of NICU personnel, this strongly contributes to the theory that virulent CoNS are spread by personnel. Again, this shows that strict hand hygiene is probably a crucial factor in prevention of CoNS LOS. As in chapter 2, S. epidermidis and S. haemolyticus were the most frequently isolated CoNS from LOS patients. These two species were also highly prevalent on the hands of NICU personnel. However, after vacation and within the control group, there was a notably high incidence of S. warneri. As S. warneri was not found among the blood isolates, we suggest that this is a relative harmless species in neonatal sepsis.
Chapters 4 and 5 describe the results of a longitudinal prospective study on the carriage of CoNS on both skin and in the intestine of neonates, as well as on the hands of their mothers. We observed that the proportion of multidrug-resistant CoNS on the skin of neonates increases during hospitalization from 40% to 91%. This is especially notable among S. haemolyticus; at birth 25% of all S. haemolyticus is multiresistant, but during hospitalization this rises to 100%. As in chapter 3, we found S. warneri to be the least resistant species. Interestingly, S. warneri was significantly less prevalent in the gut than other species, while
Summarizing discussion & future perspectives 125
Ch
ap
ter
7
S. haemolyticus is an efficient gut colonizer. It has been proposed that the gut may be the primary source for infecting CoNS in LOS and several studies have shown that CoNS from CoNS bacteremia in different patient populations could be found on the mucosa (11-14). Our findings indirectly support this theory, as we also found gut isolates to be more antibiotic resistant than other isolates. However, we also observed that skin colonization with antibiotic resistant CoNS in the first 72 hours after birth is a risk factor for CoNS LOS. This would suggest the skin to be the source of CoNS infection, although factors such as administration of postpartum antibiotics may also contribute to selection of CoNS in both gut and on skin. Further research on the role of different CoNS species and location and selection of CoNS is expected to lead to improved knowledge and possibly better targets for preventive measures.
As the ability to form biofilms is regarded as the most important virulence factor of CoNS, we focused on the treatment of biofilm as a measure to prevent CoNS LOS in chapters 6 and 7. To identify potential targets for immunotherapy, we performed an in silico search for staphylococcal surface proteins. Five proteins were chosen for further investigation. We raised rabbit-antibodies against these five proteins and tested the effect of these antibodies to assess the involvement of the respected proteins and their importance in S. epidermidis biofilm formation. Antibodies against SesC reduced the fibrinogen-binding ability of S. epidermidis, inhibited in vitro biofilm formation and significantly reduced the number of bacteria in a one day old in vivo biofilm as well as colonization and infection in a mouse jugular vein catheter infection model. Additionally, active immunization of rats in a rat model for catheter-related infections with recombinant SesC led to a 20-fold reduction of biofilm on implemented catheters. Unfortunately, the exact function of SesC is still unknown, although we have established that gene and protein expression of SesC occurs mainly during the late phase of biofilm formation. SesC is a promising target for the prevention and treatment of S. epidermidis biofilms. However, it is yet unknown if SesC also plays a role in biofilm formation of other CoNS. Although S. epidermidis is the most frequently isolated species in CoNS LOS, other species, like S. haemolyticus, are becoming increasingly important. As further studies are necessary to establish the role and prevalence of SesC in other CoNS species, the clinical application of SesC antibodies is not to be expected in the near future.
Chapter 8 gives a literature overview of potential preventive measures for CoNS LOS. Again, it is of great importance to apply strict hand hygiene measures, but several other measures may contribute to a lower incidence of CoNS LOS as well. These are mainly catheter-related strategies like the use of a vancomycin-heparin lock, the administration of cefazolin when removing a central vascular catheter and low extremity insertion of central venous catheters for total parenteral nutrition. Studies on prophylaxis with antibodies have mostly shown disappointing results, although prophylaxis with Pagibaximab seems promising (15, 16).
Future perspectives
In this thesis we aimed to identify factors which may contribute to the development of strategies to prevent CoNS LOS. As we can conclude from chapters 2, 3, 4 and 9, the most effective measure that is likely to to prevent CoNS LOS is proper hand hygiene. Not only does it decrease the number of LOS, it is also relatively cheap, compared to other prevention
Chapter 7126
strategies. In a time where health care costs are quickly rising, this is not an unimportant factor. Future strategies to prevent CoNS LOS should therefore mainly be based on hygiene education. New NICU personnel should get a proper instruction on hand hygiene when starting with their new position. Furthermore, personnel should get regular refreshment courses, to keep them triggered.
Although a proper hand hygiene is probably the most important and effective method for prevention of CoNS LOS, other strategies may also contribute to the prevention of CoNS LOS. Knowledge of neonatal colonization, transmission of CoNS and virulence factors, like biofilm formation, are therefore necessary. A thorough study on the colonization of both gut and skin of neonates with CoNS is needed to identify the importance of different factors in CoNS LOS. Special attention should be given to the role of different CoNS species, as well as the influence of host genetics and early treatment of neonates.
Immune enhancement strategies like the administration of intravenous immunoglobulins and monoclonal antibodies against specific staphylococcal targets have not shown clinically relevant effects as yet and it is highly doubtful whether antibody prophylaxis will ever be cost-effective. Antibody-based strategies may however be feasible when used to target biofilms. As we have shown in chapters 6 and 7, the surface-exposed protein SesC may be a suitable target for antibody based strategies. Future studies on this protein should focus on identifying the function of the protein, not only in S. epidermidis, but also in other CoNS when present. Futhermore, our in vitro and in vivo biofilm studies should be repeated with other CoNS species.
In chapter 8, several other preventive strategies are mentioned in Table 1. Although the effect of each of them on itself may not lead to a large decrease in CoNS LOS, implementing a combination of these strategies in daily work on the NICU may add to a significant decrease. A randomized placebo-controlled trial studying the effect of a combination of these strategies should therefore be considered.
In summary, this thesis adds to our knowledge of transmission of CoNS on NICUs and to our knowledge of biofilm formation of S. epidermidis. Knowledge of these two aspects of CoNS infection in neonates are expected to contribute to preventive strategies to reduce the high number of CoNS infections on NICUs.
Summarizing discussion & future perspectives 127
Ch
ap
ter
7
References
1. Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285-291.
2. Gray JE, Richardson DK, McCormick MC, Goldmann DA. Coagulase-negative staphylococcal bacteremia among very low birth weight infants: relation to admission illness severity, resource use, and outcome. Pediatrics. 1995;95:225-230.
3. De Giusti M, Pacifico L, Tufi D, et al. Phenotypic detection of nosocomial mecA-positive coagulase-negative staphylococci from neonates. J Antimicrob Chemother. 1999;44:351-358.
4. Krediet TG, Mascini EM, van Rooij E, et al. Molecular epidemiology of coagulase-negative staphylococci causing sepsis in a neonatal intensive care unit over an 11-year period. J Clin Microbiol. 2004;42:992-995.
5. Raimundo O, Heussler H, Bruhn JB, et al. Molecular epidemiology of coagulase-negative staphylococcal bacteraemia in a newborn intensive care unit. J Hosp Infect. 2002;51:33-42.
6. Monsen T, Karlsson C, Wistrom J. Spread of clones of multidrug-resistant, coagulase-negative staphylococci within a university hospital. Infect Control Hosp Epidemiol. 2005;26:76-80.
7. Vermont CL, Hartwig NG, Fleer A, et al. Persistence of clones of coagulase-negative staphylococci among premature neonates in neonatal intensive care units: two-center study of bacterial genotyping and patient risk factors. J Clin Microbiol. 1998;36:2485-2490.
8. Boisson K, Thouverez M, Talon D, Bertrand X. Characterisation of coagulase-negative staphylococci isolated from blood infections: incidence, susceptibility to glycopeptides, and molecular epidemiology. Eur J Clin Microbiol Infect Dis. 2002;21:660-665.
9. Cimiotti JP, Wu F, Della-Latta P, Nesin M, Larson E. Emergence of resistant staphylococci on the hands of new graduate nurses. Infect Control Hosp Epidemiol. 2004;25:431-435.
10. Duncan IB, Collins AM, Neelin EM, Roy TE. Nasal carriage of staphylococcus pyogenes by student nurses. Can Med Assoc J. 1957;77:1001-1009.
11. Eastick K, Leeming JP, Bennett D, Millar MR. Reservoirs of coagulase negative staphylococci in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1996;74:F99-104.
12. Herwaldt LA, Hollis RJ, Boyken LD, Pfaller MA. Molecular epidemiology of coagulase-negative staphylococci isolated from immunocompromised patients. Infect Control Hosp Epidemiol. 1992;13:86-92.
13. Matsuda J, Hirakata Y, Iori F, et al. Genetic relationship between blood and nonblood isolates from bacteremic patients determined by pulsed-field gel electrophoresis. J Clin Microbiol. 1998;36:3081-3084.
14. Wade JC, Schimpff SC, Newman KA, Wiernik PH. Staphylococcus epidermidis: an increasing cause of infection in patients with granulocytopenia. Ann Intern Med. 1982;97:503-508.
15. Shah PS, Kaufman DA. Antistaphylococcal immunoglobulins to prevent staphylococcal infection in very low birth weight infants. Cochrane Database Syst Rev. 2009:CD006449.
16. Weisman LE, Thackray HM, Steinhorn RH, et al. A randomized study of a monoclonal antibody (pagibaximab) to prevent staphylococcal sepsis. Pediatrics. 2011;128:271-279.
Chapter 7128
Nederlandse samenvatting
Coagulase-negatieve stafylokokken (CoNS) zijn wereldwijd de belangrijkste oorzaak van late-onset sepsis (LOS), sepsis die optreedt na 72 uur na geboorte. Alhoewel CoNS LOS een lage mortaliteit heeft, leidt de hoge morbiditeit tot een verlengde opnameduur in het ziekenhuis en daarmee tot hogere kosten voor de gezondheidszorg. Aangezien de resistentie van CoNS tegen antibiotica toeneemt, wordt preventie van infecties door CoNS steeds belangrijker.
In hoofdstuk 2 hebben we de incidentie van CoNS LOS op een grote neonatale intensive care unit (NICU) beschreven en identificeerden we patiëntkarakteristieken die geassocieerd zijn met LOS. Ook hebben we in dit hoofdstuk het gebruik van restriction fragment end labelling (RFEL) als methode voor epidemiologisch moleculaire typering van CoNS geëvalueerd. Uit 70% van de bloedkweken van very low birth weight (VLBW) neonaten met bloedbaan infecties werden CoNS geïsoleerd. Aangezien de opnameduur van meer dan 14 dagen significant geassocieerd was met CoNS LOS (odds ratio 6.0, mediane leeftijd op eerste infectiedag 8 dagen), is het duidelijk dat CoNS LOS een probleem is op de NICU. S. epidermidis en S. haemolyticus waren de meest prevalente species en 32% van alle isolaten behoorden tot één genetisch cluster. Dit resultaat was vergelijkbaar met andere studies, hetgeen suggereert dat kruiscontaminatie, bijvoorbeeld door inadequate handhygiëne, een belangrijke rol bij CoNS infecties kan hebben. Daarnaast hebben we aangetoond dat minder dan de helft van de isolaten in vitro biofilm produceerden, terwijl het overgrote deel van de geïsoleerde stammen resistent bleek tegen antibiotica. De PCR op het mecA-gen was positief in 87%, 77% was resistent tegen meerdere antibiotica. Pulsed-field gel electrophoresis (PFGE) is de meest gangbare techniek voor de moleculaire typering van CoNS. Wij hebben RFEL als alternatieve methode gevalideerd door de overeenstemming tussen RFEL en PFGE te bepalen. Deze twee technieken bleken redelijk goed overeen te komen met een significante r-waarde van 74%.
In hoofdstuk 3 onderzochten we CoNS dragerschap op de handen van NICU personeel. Eerdere studies hadden al aangetoond dat de dragerschap voor antibiotica resistente CoNS bij ziekenhuispersoneel toeneemt naarmate men langer in dienst is. Wij hebben eveneens aangetoond dat dragerschap van antibiotica resistente CoNS afneemt na een periode van afwezigheid uit de ziekenhuisomgeving. Daarnaast zagen wij dat 90% van de bloedisolaten ook op de handen van NICU personeel konden worden gevonden. Deze twee bevindingen dragen sterk bij aan de theorie dat virulente CoNS verspreid worden door personeel. Dit toont nogmaals aan dat strikte handhygiëne waarschijnlijk een cruciale factor is in de preventie van CoNS LOS. Evenals in hoofdstuk 2 werden S. epidermidis en S. haemolyticus het meest frequent geïsoleerd bij LOS patiënten. Deze twee species waren ook zeer frequent aanwezig op de handen van NICU personeel. Echter, na vakantie en bij de controle groep was er een groot aandeel van S. warneri. Aangezien we geen S. warneri vonden bij de bloedisolaten, concluderen we dat dit een relatief onschadelijke species is die niet van belang is bij de pathogenese van neonatale sepsis.
In hoofdstukken 4 en 5 beschrijven we de resultaten van een longitudinale, prospectieve studie naar de dragerschap van CoNS op de huid en in de darmen van neonaten, evenals op de handen van hun moeders. We zagen dat het aandeel van multiresistente CoNS op de huid van neonaten toeneemt van 40% naar 91% gedurende de opname. Dit was met
Nederlandse samenvatting & toekomstperspectieven 129
Ch
ap
ter
7
name duidelijk bij S. haemolyticus. Bij de geboorte was 25% van alle S. haemolyticus isolaten multiresistent, maar gedurende opname nam dit toe tot 100%. Evenals in hoofdstuk 3 was S. warneri de minst antibioticum resistente species. Opmerkelijk genoeg was S. warneri minder prevalent in de darm dan andere species, terwijl S. haemolyticus vaak gevonden werd in de darm. Er zijn theorieën dat de darm de primaire bron is van CoNS die LOS veroorzaken. Een aantal studies in verschillende patiëntenpopulaties hebben aangetoond dat CoNS die geïsoleerd waren bij CoNS bacteriemieën aanwezig waren bij kweken van mucosa. Onze resultaten ondersteunen dit indirect, aangezien darmisolaten in onze studie resistenter waren dan isolaten uit andere kweeklocaties. Echter, we zagen ook dat het gekoloniseerd zijn met antibiotica resistente CoNS in de eerste 72 uur na geboorte een risicofactor was voor het ontwikkelen van CoNS LOS. Dit suggereert dat de huid de bron van CoNS infectie is, alhoewel factoren als toediening van postpartum antibiotica ook kunnen bijdragen aan de selectie van CoNS in zowel de darmen als op de huid. Meer onderzoek naar de rol van verschillende CoNS species en de locatie en selectie van CoNS zal kunnen leiden tot aanknopingspunten voor betere preventie.
Aangezien het vermogen om biofilms te produceren als de belangrijkste virulentiefactor van CoNS wordt gezien, bestudeerden wij in hoofdstuk 6 en 7 het behandelen van de biofilm als preventiemaatregel voor CoNS LOS. Ter identificatie van mogelijke aangrijpingspunten voor immunotherapie, voerden wij een in silico analyse uit naar oppervlakte-eiwitten van stafylokokken. We kozen vijf eiwitten voor nadere analyse. Tegen deze vijf eiwitten hebben we konijnen-antistoffen opgewekt en met deze antistoffen hebben we de betrokkenheid en belang van de eiwitten in S. epidermidis biofilm vorming bestudeerd. Antistoffen tegen SesC verminderden de fibrinebindingscapaciteit van S. epidermidis, remden de in vitro biofilm vorming en zorgden voor een significante reductie van het aantal bacteriën in een één dag oude in vivo biofilm. Daarnaast zorgden de antistoffen tegen SesC ook voor de reductie van kolonisatie en infectie in een vena jugularis-katheter infectie muismodel. Tevens leidde actieve immunisatie met recombinant SesC van ratten in een rat model voor katheter gerelateerde infecties tot een 20-voudige reductie van biofilm op de ingebrachte katheters. Helaas is de precieze functie van SesC nog onbekend, alhoewel we hebben aangetoond dat de gen- en eiwitexpressie van SesC met name gedurende de late fase van biofilm vorming plaatsvindt. SesC is een veelbelovend aangrijpingspunt voor de preventie en behandeling van S. epidermidis biofilms. Het is echter onduidelijk of SesC ook een rol speelt in de vorming van biofilms van andere CoNS. Alhoewel S. epidermidis de frequentst geïsoleerde species in CoNS LOS is, worden andere species, zoals S. haemolyticus, steeds belangrijker. Aangezien meer onderzoek noodzakelijk is om de rol en de prevalentie van SesC in andere CoNS species bepalen, is het niet te verwachten dat antistoffen tegen SesC een klinische toepassing zullen vinden in de nabije toekomst.
Hoofdstuk 8 geeft een overzicht van de literatuur ten aanzien van mogelijke preventieve maatregelen voor CoNS LOS. Strikte handhygiëne is van groot belang, maar enkele andere maatregelen kunnen ook bijdragen aan een lagere incidentie van CoNS LOS. Dit zijn met name catheter-gerelateerde maatregelen, zoals het gebruik van een vancomycine-heparine lock, het toedienen van cefazoline wanneer de centrale lijn wordt verwijderd en het plaatsen van centraal veneuze katheters voor totale parenterale voeding in de lagere extremiteiten. Studies naar profylaxe met antistoffen tonen overwegend teleurstellende resultaten, alhoewel profylaxe met Pagibaximab veelbelovend lijkt.
Chapter 7130
Toekomstperspectieven
Dit proefschrift had als doel het identificeren van factoren die mogelijk bijdragen aan het ontwikkelen van maatregelen om CoNS LOS te voorkomen. Zoals we uit hoofdstukken 2, 3, 4 en 9 kunnen opmaken, is een goede handhygiëne waarschijnlijk de meest effectieve maatregel om CoNS LOS te voorkomen. Niet alleen zorgt het voor een daling van het aantal LOS, het is, vergeleken met veel andere preventieve maatregelen, ook relatief goedkoop. In een tijd waarin de kosten van de gezondheidszorg snel toenemen, is dit geen onbelangrijke eigenschap. Toekomstige maatregelen om CoNS LOS te voorkomen zouden zich daarom voornamelijk op scholing ten aanzien van hygiëne moeten richten. Nieuw personeel op de NICU zou een adequate handhygiëne instructie moeten krijgen wanneer gestart wordt met de nieuwe functie. Daarnaast zou het personeel regelmatig opfriscursussen moeten krijgen om scherp te blijven.
Alhoewel een goede handhygiëne waarschijnlijk de belangrijkste en meest effectieve maatregel is om CoNS LOS te voorkomen, kunnen andere maatregelen ook bijdragen aan het voorkomen van CoNS LOS. Kennis van de neonatale kolonisatie, transmissie van CoNS en virulentiefactoren zoals biofilmvorming, zijn daarom noodzakelijk. Een grondig onderzoek naar de kolonisatie door CoNS van zowel de darm als de huid van neonaten is nodig om het belang van verschillende factoren in CoNS LOS te identificeren. De rol van verschillende CoNS species verdient hierin bijzondere aandacht, evenals de invloed van gastheer genetica en de vroege behandeling van neonaten.
Immuunondersteunende maatregelen als het toedienen van intraveneuze immunoglobulinen of monoclonale antistoffen tegen specifieke antigenen van stafylokokken hebben tot dusver geen klinisch relevante effecten getoond en het is discutabel of profylaxe met antistoffen ooit kosteneffectief zal zijn. Maatregelen op basis van antistoffen kunnen echter mogelijk zijn als deze gericht zijn op biofilms. Zoals we in hoofdstukken 6 en 7 hebben laten zien kan het oppervlakte-eiwit SesC een geschikt antigen zijn voor maatregelen op basis van antistoffen. Toekomstig onderzoek naar dit eiwit zou zich moeten richten op het identificeren van de functie van het eiwit, niet alleen in S. epidermidis, maar ook in andere CoNS. Verder zouden onze in vitro en in vivo studies herhaald dienen te worden met andere CoNS species.
In hoofdstuk 8 staan enkele andere preventieve maatregelen genoemd in Tabel 1. Alhoewel het effect van elk van deze maatregelen op zichzelf niet noodzakelijkerwijs zal leiden tot een grote afname in CoNS LOS, kan de implementatie van een combinatie van deze maatregelen in de dagelijkse routine op de NICU bijdragen aan een significante daling van CoNS LOS. Een gerandomiseerde, placebo-gecontroleerde studie naar het effect van een combinatie van deze maatregelen zou daarom overwogen moeten worden.
Samengevat draagt dit proefschrift bij aan onze kennis van transmissie van CoNS op NICU’s en aan onze kennis over biofilm vorming van S. epidermidis. We verwachten dat kennis van deze twee aspecten van CoNS infectie in neonaten bijdraagt aan preventieve maatregelen om het hoge aantal CoNS infecties op NICU’s te verminderen.
Appendix134
Contributing authors
Peter Burghout, PhD Department of Pediatrics, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands.
Prof. Johan van Eldere, MD, PhD Laboratory of Clinical Bacteriology and Mycology, Department of Microbiology and Immunology, KULeuven, U.Z.Gasthuisberg, Leuven, Belgium.
Silvia Estevão, BSc Department of Pediatrics, Erasmus MC-Sophia Children’s Hospital, Rotterdam, The Netherlands.
Wil H. Goessens, PhD Department of Medical Microbiology and Infectious Diseases, Erasmus MC-Sophia Children’s Hospital, Rotterdam, The Netherlands.
Prof. Ronald de Groot, MD, PhD Department of Pediatrics, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands.
Prof. Peter W.M Hermans, PhD Department of Pediatrics, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands.
Deborah Horst-Kreft, BSc Department of Medical Microbiology and Infectious Diseases, Erasmus MC-Sophia Children’s Hospital, Rotterdam, The Netherlands.
Alike Kamerbeek, MD Department of Pediatrics, Sint Franciscus Hospital, Rotterdam, The Netherlands.
Ladan Khodaparast, MSc Laboratory of Clinical Bacteriology and Mycology, Department of Microbiology and Immunology, KULeuven, U.Z.Gasthuisberg, Leuven, Belgium.
Laleh Khodaparast, MSc Laboratory of Clinical Bacteriology and Mycology, Department of Microbiology and Immunology, KULeuven, U.Z.Gasthuisberg, Leuven, Belgium.
René F Kornelisse, MD, PhD Department of Pediatrics, Erasmus MC-Sophia Children’s Hospital, Rotterdam, The Netherlands.
Contributing authors 135
Ap
pe
nd
ix
Soňa Kucharíková, PhD Department of Molecular Microbiology, Flanders Interuniversity Institute for Biotechnology and Laboratory of Molecular Cell Biology, Institute of Botany and Microbiology, KULeuven, Leuven, Belgium.
Rita Merckx, BSc Laboratory of Clinical Bacteriology and Mycology, Department of Microbiology and Immunology, KULeuven, U.Z.Gasthuisberg, Leuven, Belgium.
Alewijn Ott, MD, PhD Laboratory for Infectious Diseases, Groningen, The Netherlands.
Mohammad Shahrooei, PhD Laboratory of Clinical Bacteriology and Mycology, Department of Microbiology and Immunology, KULeuven, U.Z.Gasthuisberg, Leuven, Belgium.
Marcel Sluijter, MScDepartment of Pediatrics, Erasmus MC-Sophia Children’s Hospital, Rotterdam, The Netherlands.
Benoit Stijlemans, PhD Laboratory of Cellular and Molecular Immunology, Vrije Universiteit Brussel , and Laboratory of Myeloid Cell Immunology, Flanders Interuniversity Institute for Biotechnology, Brussels, Belgium.
Appendix136
Dankwoord
En dat was het dan… Na 10 jaar is het eindelijk voorbij en is het tijd om een ieder die aan dit boekje heeft meegewerkt te bedanken. Uiteraard ga ik zeker mensen vergeten, maar ik zal een moedige poging doen om volledig te zijn…
Allereerst wil ik de ouders (en kinderen) bedanken die belangeloos toestemming hebben geleverd om hun toch al zo fragiele kinderen te samplen en te fotograferen. Kinderen die geen CoNS kunnen gebruiken naast alle ellende die ze al hebben als ze op de NICU liggen. De reden dat ze op de NICU terecht komen is meestal niet vermijdbaar, een CoNS sepsis is dat waarschijnlijk meestal wel. Laten we hopen dat we met dit werk bij kunnen dragen aan een vermindering daarvan.
En dan mijn promotoren. Prof. dr. de Groot, beste Ronald, alhoewel je het CoNS project toch vooral vanaf de zijlijn hebt begeleid, ben jij degene geweest dankzij wie ik in de wetenschap ben gerold. In 2001 heb ik in het blok kindergeneeskunde contact met je gezocht voor mijn afstudeeronderzoek en daarna ben ik eigenlijk gewoon blijven hangen. Je bent een inspiratie geweest als docent, arts, wetenschapper en mens en ik ben er trots op om bij jou te mogen promoveren
Prof. dr. Hermans, beste Peter, 10 jaar geleden heb je me “het mes op de keel gelegd” (je eigen woorden!) en me gestrikt voor dit onderzoek. Het is waarschijnlijk anders gelopen dan we beiden destijds hadden gedacht en alhoewel ik me in de afgelopen jaren wel eens heb afgevraagd of het wel een slimme keuze was om dit onderzoek te gaan doen, ben ik er achteraf gezien toch wel heel erg blij om. Ik heb in die jaren op het lab ontzettend veel geleerd, zaken waar ik tijdens mijn co-schappen en in mijn werk daarna veel profijt van heb gehad. Daarnaast droegen jouw gevoel voor humor, enthousiasme en directheid bij aan een hele prettige sfeer, zelfs op afstand. Ik kan me nog herinneren dat ik de eerste versie van het allereerste manuscript dat ik had geschreven van je terug kreeg. “Beyond repair” stond in grote rode letters op de voorkant. Ik hoop dat ik inmiddels wel wat beter schrijf.
Co-promotor dr. Kornelisse, beste René, toen we aan het project begonnen zei je dat het in 3 jaar wel af zou zijn. Het is ietsje langer geworden, maar we hebben toch wel een hele gezellige tijd gehad. Nadat Peter en Ronald toch al vrij snel naar Nijmegen waren vertrokken, was ik op jou aangewezen voor mijn begeleiding en dat heeft niet verkeerd uitgepakt. We hebben samen aardig wat verschillende dingen gedaan, van het midden in de nacht kweken van duimen en voetjes op de NICU tot het infecteren van ratten. Mede door je vriendelijkheid, gezelligheid en ook aandacht voor mijn persoonlijke leven, heb ik altijd prettig met je samengewerkt. Ik hoop dat dat met het afronden van mijn boekje niet ophoudt.
En over vertrekken gesproken, ik moet natuurlijk de persoon die er eigenlijk de oorzaak van is dat ik aan dit project ben begonnen niet vergeten, dr. Adrian! Dear Peter, unfortunately you went back to South-Africa soon after the start of my project. And I say unfortunately, not just because I lost a supervisor, but also because we got along quite well on a personal level. I hope life is treating you well!
Dankwoord 137
Ap
pe
nd
ix
Prof. van der Heijden, prof. Verbrugh en prof. Habbema, dank voor het kritisch doorlezen van het manuscript, het prettige contact in de afgelopen tijd en het plaatsnemen in de kleine commissie.
Prof. Weisman, thank you for coming, it is an honour to have you all the way from Texas for my opposition. Prof. van Eldere, na onze jarenlange samenwerking met twee mooie papers als resultaat, ben ik blij u tegenover mij te mogen vinden in de grote commissie. Dr. van Tiel, beste Frank, erg leuk dat je deelneemt aan de oppositie! Ik wil je ook bedanken voor de mogelijkheden die je mij hebt gegeven om naast mijn opleiding mijn proefschrift af te ronden. Natuurlijk ook dank aan prof. Zimmerman en dr. Fleer voor jullie deelname aan de grote commissie.
Sebas! Het is af! Al 10 jaar heb je tijdens onze uurtjes in de sportschool moeten aanhoren hoe het met mijn onderzoek zat. We hebben in de afgelopen 23 jaar veel hoogte- en dieptepunten met elkaar gedeeld en ik hoop dat we er nog veel zullen gaan delen. Ik ben blij dat je ook weer bij dit hoogtepunt naast me staat. Archana, mijn andere paranimf en geweldige zusje! Zo eens in de zoveel tijd vroeg je me wat ik nou precies ook alweer onderzocht, ik hoop dat dat nu een beetje duidelijk is geworden. Ik heb je in de afgelopen 10 jaar zien opgroeien tot een vrouw, die heeft laten zien hoe sterk ze is en dat ze van alles kan bereiken, ondanks alles wat er op haar pad komt. En dat met een heerlijke gevoel voor humor! Ik ben ontzettend trots op je.
En dan de collega’s! Zoals eerder gezegd ga ik ongetwijfeld mensen vergeten, alvast mijn excuses daarvoor. Laat ik beginnen bij de collega’s van de kinderinfectieziekten. Allereerst natuurlijk Marcel, een half jaar na start wees Peter je aan het CoNS project toe en ik had me geen betere ondersteuning kunnen wensen. Niet alleen heb je bergen werk verzet, ook dacht je altijd actief mee en was je een ontzettend fijn persoon om mee samen te werken. Na het vertrek van de twee Peters heb je mij, een verdwaalde medicus, eigenlijk verder begeleid op het lab. Dank je voor alles, zonder jou was dit boekje niet wat het nu is. Silvia, altijd goedlachs en sarcastisch, dank je voor je vele biofilmplaten en natuurlijk de gezelligheid op onze kamer en daarbuiten. Ook Theo enorm bedankt voor je eiwithulp en uiteraard voor alle humor waar je het lab mee opfleurt. De promovendi die mij voor gingen, Debbie en Marieke, ik heb veel van jullie geleerd! Wouter, drie maanden voor mij begonnen, drie jaar voor mij geëindigd. Dank je voor de ontzettend leuke tijd samen! Kees, het was leuk je nog even meegemaakt te hebben en dank je dat je mij in de afgelopen jaren gefaciliteerd hebt, ook al was ik al lang en breed “weg”. Natuurlijk ook dank aan mijn studenten, Brenda en Laurie, voor al jullie werk. Ook dank aan de infectiecollega’s in het Sophia: Nico, Gert-Jan, Michiel, Pieter, Annemarie en iedereen waar ik nu even snel niet op kom. Het leven van een promovendus is nog veel zwaarder zonder adequate managementsondersteuning, ik ben Ada, Conne en Ingrid L dan ook veel dank schuldig voor de meer dan adequate hulp die jullie in de afgelopen jaren hebben verleend. Een deel van de kinderinfectieziekten in Rotterdam is verhuisd naar Nijmegen, dus ook een dank aan alle collega’s aldaar. Met name Saskia, Peter B, Angela (inmiddels terug in Leiden) en Christa wil ik toch even noemen.
Ik ga me er niet aan wagen om alle overige collega’s van het Laboratorium Kindergeneeskunde bij naam te bedanken, want dan weet ik zeker dat ik mensen ga vergeten. Dus dank aan alle promovendi, analisten, postdocs en bazen van de neo, onco en gastro voor de samenwerking
Appendix138
en vooral de gezelligheid. Ondanks dat we met hele verschillende groepen op het lab zaten, was daar niets van te merken in het onderlinge contact, in ieder geval niet op onze (in meerdere opzichten) multiculti kamer. En natuurlijk zal ik alle legendarische labuitjes en kerstvieringen ook niet vergeten…
Dank ben ik ook verschuldigd aan de mensen van de afdeling Neonatologie in het Sophia. Prof. van Goudoever, beste Hans, inmiddels ben jij ook weg, maar dank je dat je me destijds alle ruimte hebt gegeven om mijn studies “tussendoor” uit te voeren op jouw afdeling. Ook wil ik alle neonatologen en verpleegkundigen, die me geholpen hebben door op de raarste tijdstippen hun duim op een kweekplaat te duwen of poep voor me te verzamelen, bedanken voor hun inzet.
I shouldn’t forget the colleagues from Leuven! Mohammad, we worked closely together in the past years, resulting in two very nice papers and your thesis. Thank you for the collaboration and the conversations.
En toen ging ik naar Maastricht… Ik weet dat ik heel vaak roep dat dingen in Rotterdam beter zijn, maar er is één iets dat volgens mij toch echt niet beter kan dan in Maastricht en dat is onze AIOS groep. Guy, Robin, Michiel, Fleur en sinds kort ook Elly, ik had het niet beter kunnen treffen dan met jullie. En natuurlijk horen de infectie-AIOS Jaclyn en Chantal ook in dat rijtje thuis. Het is niet alleen hartstikke gezellig op onze veel te drukke kamer, ook qua collegialiteit zijn jullie top. En niet alleen met de AIOS, ook met de bazen, Antoinette, Astrid, Danielle, Dirk, Frank, Helke, Inge, Luis, Paul, Petra en Selwyn, is het ontzettend prettig werken. De gezelligheid wordt compleet gemaakt door alle analisten van de bacterio, viro en moleculaire en alle mensen van het secretariaat, ZIP, en de research “aan de overkant”. Helaas heb ik ook al een paar mensen weg zien gaan. Kitty, jammer dat je ging, maar ik ben blij dat we nog steeds onze projectjes samen hebben lopen. Cathrien, dank voor alle gezellige praatjes. Valère, jouw vertrek was natuurlijk een enorme aderlating voor de afdeling. Je kennis, gevoel voor humor en prettige persoonlijkheid worden gemist. En over aderlatingen gesproken, Ellen, jouw vertrek laat grote leegte achter, maar gelukkig ben ik nog op tijd een project met je gestart. En nog bedankt voor het steeds vragen hoe het met mijn proefschrift was, het heeft zeker geholpen!
In de afgelopen 10 jaar hebben veel vrienden ervoor gezorgd dat ik het ook buiten het werk om naar mijn zin had. Uiteraard wil ik iedereen bedanken, maar om niemand te vergeten ga ik niet iedereen noemen. Een aantal hebben echter direct of indirect ook bijgedragen aan dit boekje. Gijs, toen ik 10 jaar geleden moest bedenken of ik dit project wilde doen, hebben mijn gesprekken met jou invloed gehad op mijn keuze. Het is jammer dat we beiden voor de meest perifere locaties in Nederland hebben gekozen voor onze specialisatie, maar ik ben blij dat we desondanks nog steeds regelmatig contact hebben. Waheeda en Anjali R, jullie bedankt voor het faciliteren, ik heb er tot op het laatste moment gebruik van gemaakt. Jullie weten wel waar ik het over heb.
Voor familie geldt eigenlijk hetzelfde als voor vrienden, zij hebben ervoor gezorgd dat mijn tijd buiten het werk erg leuk was. Het is ook geen toeval dat die twee groepen soms in elkaar gingen overlopen. Hoe dan ook, ook hier geen namen, maar wel dank, vooral aan alle mensen van de Hira en Baldew uitjes. Jullie weten wie jullie zijn!
Dankwoord 139
Ap
pe
nd
ix
En dan mijn ouders. Ma en pa, hij is eindelijk af hoor! Toen ik 10 jaar geleden vertelde dat ik nog niet aan mijn co-schappen ging beginnen, vroegen jullie je af of dat wel een goed idee was. Desondanks hebben jullie mij altijd gesteund en de vrijheid gegeven om te doen wat ik dacht dat het beste was. Ik heb het altijd ontzettend gezellig gehad bij jullie en ondanks dat ik nu een paar uur verderop woon is het toch altijd weer als vanouds als ik terug ben. Jullie hebben me zo vaak gevraagd of ik nu eindelijk eens ging promoveren, ik kan niet anders dan dit boekje aan jullie opdragen.
Lieve Aartie, jarenlang was ik bezig met dit project, toen ging ik met jou trouwen en was het boekje ook ineens af. Dat kan geen toeval zijn, jij haalt het beste in mij naar boven. Van het onderzoek heb je heel weinig meegekregen, van de afronding des te meer. Pas getrouwd en dan blijkt je man ineens uren achter de computer te gaan zitten om een boekje te maken. Gelukkig is dat voorbij, we gaan nu samen nieuwe spannende uitdagingen tegemoet! Ik hou van je!
Appendix140
List of publications
Hira V, Kornelisse RF, Sluijter M, Kamerbeek A, Goessens WH, de Groot R, Hermans PW. Colonization dynamics of antibiotic resistant coagulase-negative staphylococci in neonates. J Clin Microbiol. 2013;51:595-597. Epub 2012/11/09.
Hira V*, Shahrooei M*, Khodaparast L, Stijlemans B, Kucharikova S, Burghout P, Hermans PW, van Eldere J. Vaccination with SesC Decreases Staphylococcus epidermidis Biofilm Formation. Infect Immun. 2012;80(10):3660-8. Epub 2012/07/18. (*Equal contributions)
Hira V, Sluijter M, Goessens WH, Ott A, de Groot R, Hermans PW, Kornelisse RF. Coagulase-negative staphylococcal skin carriage among neonatal intensive care unit personnel: from population to infection. J Clin Microbiol. 2010;48(11):3876-81. Epub 2010/09/10.
Shahrooei M, Hira V, Stijlemans B, Merckx R, Hermans PW, van Eldere J. Inhibition of Staphylococcus epidermidis biofilm formation by rabbit polyclonal antibodies against the SesC protein. Infect Immun. 2009;77(9):3670-8. Epub 2009/06/17.
Hira V, Sluijter M, Estevao S, Horst-Kreft D, Ott A, de Groot R, Hermans PW, Kornelisse RF. Clinical and molecular epidemiologic characteristics of coagulase-negative staphylococcal bloodstream infections in intensive care neonates. Pediatr Infect Dis J. 2007;26(7):607-12. Epub 2007/06/29.
van de Sande WW, Janse DJ, Hira V, Goedhart H, van der Zee R, Ahmed AO, Ott A, Verbrught H, van Belkum A. Translationally controlled tumor protein from Madurella mycetomatis, a marker for tumorous mycetoma progression. J Immunol. 2006;177(3):1997-2005. Epub 2006/07/20.
van Kampen JJ, Fraaij PL, Hira V, van Rossum AM, Hartwig NG, de Groot R, Luider TM. A new method for analysis of AZT-triphosphate and nucleotide-triphosphates. Biochem Biophys Res Commun. 2004;315(1):151-9. Epub 2004/03/12.
Curriculum vitae 141
Ap
pe
nd
ix
Curriculum vitae
Vishal Hira was born on August 29th 1979 in Paramaribo, Surinam. Only eight months old, he moved with his parents to Schiedam in The Netherlands. After primary school, he attended grammar school at the Stedelijk Gymnasium Schiedam, where he graduaded in 1997. In the same year he started medical school at the Erasmus University Rotterdam. In 2001 he attended an internship surgery at the Hacettepe University Hospital in Ankara, Turkey (supervisor: Dr. O. Abbassoğlu). Vishal received his doctoraal in 2002, after his research project at the Department of Pediatric Infectious Diseases of the Erasmus MC on the use of MALDI-TOF mass spectrometry to measure intracellular triphosphates (supervisors: Dr. P.L.A. Fraaij, Dr. T.M. Luider and Prof.dr. R. de Groot). During the next few months, he performed research on single-nucleotide polymorphisms in meningococcal sepsis at the Laboratory of Pediatrics (supervisors: Dr. M. Emonts and Prof.dr. P.W.M. Hermans) and on timeliness of vaccinations at Vaxinostics B.V. (supervisor: Dr. H.C. Rümke). Eventually, he started as a PhD student at the Department of Pediatric Infectious Diseases/Laboratory of Pediatrics from 2003 to 2007 (supervisors: Prof.dr. P.W.M. Hermans, Dr. R.F. Kornelisse and Prof.dr. R. de Groot). In 2008 he started the clinical phase of his medical studies and in 2009 Vishal graduated medical school. Subsequently he worked as a physician at the department of internal medicine at the Maasstad Hospital in Rotterdam. Since 2011, Vishal is a resident in medical microbiology at the Maastricht University Medical Centre (supervisor: Dr. F.H. van Tiel; head: Prof.dr. P.H. Savelkoul). Since the start of his studies, Vishal has been actively involved as a member and/or co-founder of numerous committees and boards of associations and foundations. In these functions he has organized many events, ranging from small and local debates to nationwide symposia. Currently, he is member of the board of Sapna Foundation, an organization building a health clinic for the medical care and education of pregnant women, mothers and young children in Bihar, India.Vishal is married to Aartie, together they live in Maastricht.

















































































































































![eind presentatie.VCI [Compatibiliteitsmodus] - ctgnetwerk.com · 54 Literatuur 1. Alsous F Alsous F, Khamiees M, DeGirolamo A, Amoateng-Adjepong Y, Manthous CA,; Negative fluid balance](https://static.fdocuments.nl/doc/165x107/5e0db73c09e3eb35bb759fbe/eind-compatibiliteitsmodus-ctgnetwerkcom-54-literatuur-1-alsous-f-alsous-f.jpg)