B and T cell epitopes of glycoprotein D of herpes simplex virus type 1
Transcript of B and T cell epitopes of glycoprotein D of herpes simplex virus type 1
FEMS Microbiology Immunology 76 (1991) 59-68 © 1991 Federation of European Microbiological Societies 0920-8534/91/$03.50 Published by Elsevier ADONIS 092085349100060E
59
FEMSIM 00155
Review
B and T cell epitopes of glycoprotein D of herpes simplex virus type I
Sytske Welling-Wester, Albert-Jan Scheffer and Gjalt W. Welling
Rijksuniversiteit Groningen, Laboratorium voor Medische Microbiologie, Groningen, The Netherlands
Received 23 November 1990 Accepted 3 December 1990
Key words: Herpes simplex virus; Glycoprotein D; B cell epitope; T cell epitope; Synthetic peptide
1. HERPES SIMPLEX VIRUS
The herpes simplex viruses types 1 and 2 (HSV- 1 and HSV-2), also known as human herpesvirus types 1 and 2, are important human pathogens belonging to the subfamily alphaherpesviridae of the family herpesviridae. Usually they cause rela- tively benign though annoying disorders like cold sores, genital herpes, and eye infections that occa- sionally may turn serious: e.g., in the Western hemisphere, herpetic keratitis is the leading cause of unilateral blindness. Rare but severe diseases include herpetic encephalitis and esophagitis; also uncommonly but at a steadily increasing pace, HSV is the cause of severe and even life-threaten- ing disseminated infections in immunologically deficient individuals and infants [1].
Although man is probably the natural host to HSV, the viral host range covers many mam- malian species. Moreover, many established cell lines support the multiplication of HSV-1 and 2.
Correspondence to: S. Welling-Wester, Laboratorium voor Medische Microbiologie, Oostersingel 59, 9713 EZ Groningen, The Netherlands.
These circumstances enable detailed examination of both virus types in vitro and in vivo, making herpes simplex virus one of the best studied hu- man viruses second only to HIV.
All herpesviruses possess the abilities of latency and recurrence; i.e., they can reside in a latent state in certain types of host cells lifelong, and they can be provoked to resume active replication by a wide variety of trigger signals. Both primary infection and reactivation of latent HSV may lead to mucocutaneous lesions which usually resolve in immunocompetent individuals [1].
2. GLYCOPROTEIN D
The herpesviruses are enveloped DNA-viruses carrying several distinct glycoproteins on their surface. HSV-1 and HSV-2 possess at least seven different glycoproteins labelled gB, gC, gD, gE, gG, gH and gI. The existence of two additional glycoproteins is deduced from the the nucleotide sequence of the gD gene [2,3]. Glycoproteins B, D and H are essential for infection by HSV, while glycoproteins C, E and I are not. The glycopro- reins are present in the virus envelope and the
60
membranes of infected cells. Thus, they are obvi- ous targets both for the host immune response and for investigators in search of candidate vaccines against HSV. Since gD is a major immunogen during natural infection, much research has been and still is directed at definition of its antigenic properties.
The role of gD in the viral replication cycle has been partly elucidated by analysis of the effects of its removal and its blockade. Studies of gD-minus mutants showed that these are able to attach to the host cell, but that their replication is impeded soon afterwards [4].
The functions of gD can be blocked by neutral- izing antisera and monoclonal antibodies (mAbs) directed against gD. mAb-coated virions do attach to the cell membrane but cannot penetrate [5]. Also, virion gD can be outcompeted by having the aimed-for host cell constitutionally express gD through recombinant DNA [6]. Such cells are re- sistant to infection by HSV. This, and other evi- dence leads to the hypothesis that after initial attachment of a HSV particle, the binding of its gD to a specific receptor on the cell membrane is required to initiate virion penetration. Mature gD of HSV-1 and HSV-2, gD-1 and gD-2, respec- tively, have quite similar polypeptide backbones with lengths of 369 [2,7] and 368 [8,9] amino acids, respectively, with about 85% sequence identity in their extracellular portion. The three disulfide bridges probably are at identical positions [10,11] and both proteins carry three N-linked and two or more O-linked oligosaccharide residues [12,13].
3. B CELL EPITOPES OF gD IDENTIFIED BY POLYCLONAL ANTISERA AND MONO- CLONAL ANTIBODIES
3.1. Epitopes Antigenic sites and epitopes of proteins are
those regions of a protein that react with elements of the immune system2 B cell epitopes and anti- genic sites react with antisera, T cell epitopes are recognized by T cells in combination with HLA molecules. The complete structure of a B cell epitope, which provides information on all re- sidues involved in binding, can only be de-
termined by X-ray diffraction of crystals of com- plexes of Fab fragments of the antibody molecule with the antigen. The structures of five B cell epitopes elucidated by X-ray diffraction show that large areas of the molecule consisting of 15-22 amino acids are involved, encompassing two to five surface loops of the antigen [14-16]. In con- trast to the X-ray studies, which demonstrate that epitopes are conformation-dependent, several studies [17,18] suggest that B cell epitopes on protein antigens are either continuous or discon- tinuous. It is assumed that continuous epitopes consist of stretches of consecutive amino acids, varying in length from 4 to 12 amino acids, which are still recognized as antigenic sites after de- naturation of the protein. Continuous epitopes defined in this way are localized by using syn- thetic peptides according to the amino acid se- quence in the native protein. Discontinuous epi- topes, on the other hand, are defined by their susceptibility to denaturation and the involvement of different parts of the polypeptide chain, which are probably in close proximity in the native struc- ture. Probably the truth is somewhere in the mid- dle, meaning that a discontinuous epitope may consist of several linear stretches of the poly- peptide chain. Synthetic versions of these linear stretches may then be sufficient for binding of antibodies. This is in agreement with the observa- tion that small parts of discontinuous epitopes can be identified with random peptide libraries [19- 21]. The existence of continuous epitopes defined in this way may vary from protein to protein and is completely dependent on the structure of the protein and the localization of the epitopes. In the opinion of others [22] the observed continuous epitopes are mainly due to the inappropriate use of (partly) denatured proteins. Unfortunately no X-ray information on gD is available and epitopes will be discussed as discontinuous and continuous depending on their reactivity upon denaturation.
3.2. Reactivity of peptides of the N terminus and C terminus of gD with antisera from different species
The continuous epitopes of gD have been studied by several groups. Bosch [23], Cohen [24], Eisenberg [25], Minson [7], Muggeridge (reviewed in ref. 26) and Isola [27] studied the reactivity of
mAbs against gD with fragments of gD and with synthetic peptides of gD. Besides the reactivity of mAbs, the reaction of polyclonal antisera against HSV and gD obtained from different animal species was studied with synthetic gD peptides [28-30]. Polyclonal antisera from the different species were studied, including sera from rabbits either infected or immunized with HSV-1, mice immunized and subsequently infected with HSV-1, guinea-pigs infected with HSV-2, and humans either HSV seropositive or negative by ELISA and immunofluorescence assay. These polyclonal sera reacted most frequently with synthetic peptides of the N-terminal region of gD, the region adjacent to the N terminus of the transmembrane spanning region, and the cytoplasmic tail of gD. Table 1 summarizes the reaction of the sera from the above mentioned species with overlapping peptides, each 15 amino acids long, according to the sequence of
61
gD in the N terminus, and the regions adjacent to the transmembrane spanning region. All three re- gions are hydrophilic. Among the synthetic peptides of the N-terminal region, peptide 10-24 was recognized most frequently by the various antisera. Exceptions were antisera from rabbits immunized with an extract of HSV-1 infected cells, which did not react with the N-terminal synthetic peptides. Either this is due to the im- mune response to a different antigen presentation (infection vs. immunization) or to the antigen pre- paration used for immunization, which may have lost part of its N-terminal amino acid sequence during the extraction procedure due to proteolysis. Peptide 10-24 did not react with HSV-negative sera from rabbits, mice, and guinea-pigs, nor did any of the other synthetic peptides. However, peptide 10-24, and also some of the other peptides, were recognized by sera from otherwise HSV-
Table 1
Differences in reactivity of antisera from various species with synthetic peptides of gD of the N terminus, and on either site of the transmembrane region
(a) sera from rabbits either infected (n = 12) on the eye with 5 × 105 p.f.u, of HSV-1, or immunized (n = 4) three times with an extract of HSV-1 infected vero cells (1 mg each time at 4-week intervals); (b) sera from mice (n = 10) immunized four times with 2.5 × 106 p.f.u, heat-inactivated HSV-1, which survived a subsequent challenge with 109 p.f.u, of HSV-1 [29]; (c) sera from guinea-pigs (n = 7) infected with approx. 108 HSV-2 on the skin; (d) sera from healthy medical students, seropositive (n = 38) and seronegative for HSV (n = 56) as determined by ELISA and immunofluorescence assay [30]. A positive reaction is indicated by an open circle (less than 10% of the individuals positive) or by a filled circle (over 10% of the individuals positive).
PEPTIDES
e ¢ e * ¢ g g g g # # # & &
RABBITS
infected • • • • • • • • • • •
immunized • • • • • • • • •
MICE
infected and immunized • 0 0 0 • 0 • • •
GOINEA PIGS
infected • • • • • •
HUMANS
HSV-positive 0 • 0 • • 0 0 0 •
HSV-negative • 0 0 0 0 • O O
62
negative humans [30]. There are at least two ex- planations, the most likely being that there is a certain degree of similarity between the synthetic peptide and an amino acid sequence in an unre- lated protein [31,32]. For example, in peptide 10- 24, residues 12-18 are identical to a sequence in protein L1 of human papilloma virus. Another possibility is that the peptide as used in the test system exists in a particular conformation, which is recognized by antibodies not exclusively di- rected against HSV.
Peptides 260-274, 270-284 and 280-294 were recognized by sera of all species investigated. Sera from some species (mice and man) react less fre- quently with some of the peptides. Differences in reactivity among the different species were found with peptides 290-304 and 300-314. Mouse and guinea-pig sera did not react with these peptides,
while a relatively high percentage of the human sera is reactive with peptide 300-314. The guinea- pigs had been infected with HSV-2, of which especially the amino acid sequence in peptide 300-314 is more than 50% different [9] from HSV- 1, which may account for the lack of reactivity. In the cytoplasmic part of gD, comprising residues 340 to 369, peptide 355-369 reacted with antisera of all species investigated. This peptide also re- acted with a relatively large proportion of various individual sera. Differences in reactivity among the sera from the various species were found for peptides 340-354 and 348-362.
Although most of the differences in the im- mune response to gD are supposed to be the reflection of differences in the major histocompa- tibility system, in inbred mice individual dif- ferences in the reactivity with synthetic peptides
Vll • • • • I N •
Ib II XI
60 1½0
V
J2o
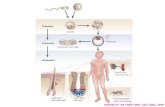
Fig. 1. Reaction of polyclonal antisera from different species with synthetic peptides of gD. The large bar on top is a schematic representation of the gD molecule, the transmembrane spanning region is indicated by a hatched block. The N-linked glycosylation sites (residues 94, 121 and 262) are indicated by filled triangles. The continuous epitopes (roman numbers) are indicated by solid lines, and involve the following regions: group VII, residues 11-19, 9-21; group II, residues 264-287, 272-279; group XI, residues 284-301 and group V, residues 340-354. Filled dots indicate m a r mutations 25, 54, 60, 72, 120, 129, 132, 140, and 216 of discontinuous epitopes. The hatched line (Ib) indicates the region of residues 234 to 275 involved in binding group Ib mAbs. Below the gD molecule are indicated the synthetic peptides investigated; solid lines meaning a positive reaction with at least one antiserum, the dotted lines indicating peptides which did not react. The data were compiled from human antisera [28,30]; rabbit anti-gD sera [27]; anti-HSV sera of infected and immunized rabbits (S. Welling-Wester, unpublished results); anti-HSV sera of immunized and infected mice [29]; anti-HSV-2 sera of infected guinea-pigs (H.J. Geerligs, unpublished results). The following peptides were included: first row, peptides 1-13, 1-14, i-23, 2-12, 9-21, 10-24, 12-21, 18-30, 20-34, 22-38, 30-44, 31-46, 38-56, 40-54, 50-64; second row, peptides 52-61, 57-77, 60-74, 63-72, 70-84, 77-98, 78-98, 80-94, 82-93, 83-92, 85-98, 90-104, 99-117, 100-114, 110-124; third row, peptides 118-137, 120-134, 121-130, 124-135, 130-139, 130-144, 137-150, 140-154, 150-159, 150-164, 151-165, 160-174, 166-180, 170-184, 180-194; fourth row, peptides 181-190, 181-197, 190-204, 198-215, 200-214, 206-215, 207-226, 210-224, 216-232, 220-234, 230-244, 232-243, 240-254, 244-267, 245-260; fifth row, peptides 250-264, 260-274, 260-284, 264-275, 267-276, 267-281,268-287, 270-284, 280-294, 282-301,284-301,288-297, 290-304, 300-314, 302-315; sixth
row, peptides 303-312, 314-323; seventh row, peptides 340-354, 340-356, 348-362, 355-369, 358-369.
are found, suggesting the influence of other fac- tors.
3.3. Reactivity of polyclonal sera with synthetic peptides of gD, covering the complete sequence
Altogether over 80 synthetic peptides of gD were investigated for reactivity with polyclonal antisera. Different investigators [27-30] used slightly different peptides and antisera from vari- ous sources (Fig. 1). A large number of synthetic peptides of gD were reactive with the antisera. Long stretches in the N terminus, in the region near the membrane spanning region and in the cytoplasmic region of gD are antigenic. Only part of the regions reacting with the polyclonal antisera correspond to those which have been defined as continuous epitopes of gD by mAbs. Several groups of mAbs against gD (groups VII, group II, group XI and group V) [24,27,33] are directed against continuous epitopes and react with syn- thetic peptides. The epitope of group VII mAbs is localized in the N terminus and defined by re- sidues 9-21 [23] and 11-19 [24]. Antigenic sites of the groups II and XI mAbs consist of several epitopes, which are partly overlapping and ad- jacent to each other in the region N-terminal to the transmembrane region, comprising residues 264-287 and residues 284-301, respectively. A much larger area, extending from residue 250 to 314, can be regarded as a potentially antigenic region (Fig. 1). Of the peptides investigated in this region, peptide 288-297, which was only tested against a limited number of human anti-HSV sera, failed to react [28].
It is proposed [11] that the regions N-terminal to the transmembrane region of glycoproteins' vi- rion-surface portions form 'stalks' between the membrane anchor and the disulphide-bonded 'head' domains. Detailed analysis of two other viral membrane glycoproteins, viz. hemagglutinin and neuraminidase of influenza virus, by a combi- nation of immunological methods and X-ray dif- fraction, showed that the epitopes were mainly located on the 'head' domains [16,34,35]. The data on the epitopes in the gD molecule so far, suggest that in this case the proposed 'stalk' portion is highly antigenic.
The epitope of group V mAbs is present in the
63
cytoplasmic tail of gD and is defined by reactivity with peptide 340-356. Taking into account the reactions of the synthetic peptides of the cyto- plasmic region with polyclonal antisera, the cyto- plasmic tail may be a large antigenic region con- sisting of several overlapping epitopes. A number of the synthetic peptides which reacted with the polyclonal antisera do not map in or near to continuous epitopes of gD. This could suggest that these regions represent continuous epitopes not yet described, but it is more likely that these peptides contribute to conformation-dependent epitopes.
The information on discontinuous epitopes of gD is limited and based either on the reaction of conformation-dependent mAbs with fragments of gD or on studies with mar (monoclonal antibody resistant) mutants of gD [36]. Four groups of mAbs (groups I, III, IV and VI) recognize discon- tinuous epitopes [26]. Most data on conformation- dependent epitopes are on the epitope of group I, subdivided in Ia and Ib [37,38]. Residues 234-275 are essential for Ib, as are residues 132 and 140, while the epitope of Ia mainly resides in the sequence of 1-233, with a mar mutation at residue 216. Synthetic peptides containing residue 216, or with overlapping sequences, may be recognized by some of the polyclonal antisera as being part of a discontinuous epitope. The antigenic site of group III mAbs consists of several epitopes. They all recognize a fragment of gD, containing residues 21-226. The contribution of residue 54 to one of the group III epitopes has been described [26]. The epitopes of groups IV and VI are HSV-1 type-specific and the information on the amino acids involved in binding is scarce. Both groups react with a 1-226 fragment of gD. A mar muta- tion of group VI is located at residue 121 [26]. In addition, unclassified mAbs have been described [5,7]; of these, AP12 and AP7 have mar mutations at residues 60 and 129, and residue 25, respec- tively [7]. Group VII is HSV type 2 specific. Residue 72 was found to be involved, since a HSV type 1 isolate containing a mutation at this re- sidue, was able to bind a type 2 specific mAb [39].
Some of the synthetic peptides, which contain a residue involved in a mar mutation, viz. residues 16, 25, 54, 60, 72, 121, 129, 132, 140, and 216,
64
react with polyclonal antisera (Fig. 1). Peptides 118-137, 120-134, 121-130 and 124-135, for ex- ample, all contain residue 129, a mar mutation site of mAb AP12 [7]. Of these peptides, only peptides 118-137 and 120-134 react with polyclonal anti- sera. This shows that not all peptides which con- tain residues involved in mar mutations react with the polyclonal antisera. Of these polyclonal sera only a small percentage of the antisera is reactive. For instance, only 30% of the mouse antisera, 16% of the rabbit antisera, and none of the human and guinea-pig sera react with peptide 120-134. This indicates that the reactivity is probably due to a small part of the sequence, consisting of a few residues essential for binding, which form a linear part of a large discontinuous epitope.
Besides peptides with mar mutation sites, peptides near glycosylation sites are reactive with the polyclonal antisera, probably because these regions are exposed in the gD molecule and easily accessible for the immune system.
There is only one region, residue 165 to 200, of which the synthetic peptides do not react with any of the antisera. This region partly overlaps re- sidues 137 to 185; the largest continuous stretch of amino acids, in which gD-1 and gD-2 are identical [9]. The lack of reactivity of the antisera suggests that the region may be buried inside the gD mole- cule, although it is not particularly hydrophobic. On the other hand, it cannot be excluded that the lack of reactivity is due the fact that this part of gD is highly involved in the folding of the mole- cule and is exclusively recognized when in its native conformation.
4. T CELL EPITOPES OF gD
The T cell mediated immune response has an important role in the protection against HSV (re- viewed by Nash et al. [40]). Human cytotoxic lymphocyte (CTL) clones recognizing gD have been isolated [41], but it has been difficult to detect gD-specific CTLs after infection or im- munization of mice: four groups reported negative results [41-46]. With modified presentation meth- ods, gD-specific murine CTLs and other T cells have now been demonstrated [47-51[ and CD4 +
and CD8 + clones with cytolytic properties have been isolated [48].
Compared to the B cell epitopes of gD, little is known about its T cell epitopes: the regions in the gD molecule activating any class of T lympho- cytes. T cell epitopes are known to consist of continuous stretches of 8-12 amino acids [52]. DeFreitas et al. [42] were first to demonstrate that synthetic peptides representing the N-terminal 23 amino acids of the gD molecule are able to activate peripheral blood T cells of HSV seropositive and -negative volunteers in vitro. This was confirmed by studies on a series of synthetic 15-mer peptides spanning the entire gD-1 molecule except the transmembrane region (Damhof, R.A. and Wel- ling-Wester, S., unpublished results): the peptide corresponding to residues 10-24 was recognized by lymphocytes from five out of seven individuals seropositive for HSV. Peptides from region 290- 314, adjacent to the membrane spanning region, were equally frequent activators of human lym- phocytes. Other regions were recognized less fre- quently but evoked high stimulation indices (> 6), especially peptides 120-184, 190-214 and 270- 294. Peptides from the region 214-270 induced a poor response or none at all. Heber-Katz et al. reported a similar response of murine T cells to the N terminus of gD [53,54]. In a following experiment, the same group [55] studied a series of 28 overlapping peptides corresponding to the en- tire extracellular portion of gD-1. Lymph node T cells from mice primed with gD-1 responded ex- clusively to peptide 241-246, while cells from HSV-1 infected animals were stimulated by 17 to 22 out of the 28 peptides tested. Wyckoff et al. [56] confirmed the importance of region 1-23 for murine T cell stimulation. In contrast to the re- sults of Yamashita et al. [55], peptide 268-287 was found to stimulate T cells; peptide 340-356, from the intracellular part of gD, was stimulatory also. The three regions were recognized by both T helper and T suppressor cells.
In conclusion, it has been firmly established that the N-terminal portion of gD carries an epi- tope recognized by human and murine T cells; for the other epitopes, scattered along the gD se- quence, there appears to be little agreement be- tween mice and man.
5. PROTECTION AND EPITOPES OF gD
The HSV particle invading an immunocompe- tent individual for the first time will encounter early barriers like macrophages, natural killer (NK) cells, Langerhans cells [57] and the interferons. In neonates it may find maternal neutralizing anti- body and antibodies mediating antibody-depen- dent cellular cytotoxicity (ADCC) in its way [58,59]. These mechanisms may limit infection to the initial inoculation site but normally will neither stop the virus from establishing latency in periph- eral ganglia, nor prevent recurrent infections.
However, the primary infection experience with HSV usually teaches the defense system to effec- tively withstand exogenous reinfections by HSV-1 and HSV-2 alike [60], giving designers of HSV vaccines confidence that it should be possible to protect against primary HSV infection, too.
A cellular mechanism of protection against re- current disease is required because reactivation generally does not result in viremia but rather in cell-to-cell spread of virus. Consequently, high anti-HSV neutralizing antibody titers do not pro- tect from recurrent disease, and may even play a negative role [61] (Scheffer, A.J. and Ebels, E.J., unpublished results). A decrease in levels of inter- ferons, interleukin-2, cell-mediated cytotoxicity and lymphokine-activated killer cells is correlated with the recurrence of HSV infections in man [62] and guinea-pigs [63]. In rabbits, T cells surround- ing neuron cell bodies appear to restrain coloniza- tion of neurons by HSV and to prevent its spread to other neural cells [64].
Despite a multitude of studies in man and protection experiments in animals, the defensive mechanisms interfering with the spread of HSV from its initial replication site, preventing recur- rent infections, and mediating recovery from clini- cal infection are still far from clear. This is partly due to the conflicting and even paradoxical data obtained by different groups.
Apart from the complexity of the matter, con- tradictory results can be partly accounted for by the peculiarities of the animal models employed and the countless differences in virus and host strains, immunization routes, adjuvants etc. More- over, evidence is accumulating that often under-
65
estimated factors like the handling and housing of animals, and their rank in the social (pecking) order affect their immune status and outcome of infection [65].
Basically, two approaches have been used to study protection: animals were either immunized with glycoproteins and fragments thereof, or pas- sively immunized by the transfer of mAbs and antisera. In this respect, the glycoproteins of HSV (for review see refs. 47, 50, 66, 67) have been extensively studied. Animals immunized with these proteins were either resistant to a challenge with a lethal dose of HSV, or the infection was restricted. Immunization with gD-1 and gD-2 combined with Freund's adjuvant protected mice against lethal HSV challenge [67,68]; without adjuvants, gD-1 protected mice from herpetic keratitis and lethal challenge with either HSV-1 and -2 [69]. This effect was not mediated by cellular immunity but rather by neutralizing antibodies and complement- dependent cytolysis [69] or by delayed hypersensi- tivity [47]. Immunization with a recombinant vac- cinia virus expressing gD-1 protected guinea-pigs from primary and recurrent HSV-2 lesions [70]; the degree of protection was related to HSV- specific T cell responses, not to neutralizing anti- body titers [71].
Despite these and a number of other studies using fragments of gD in immunization experi- ments, little information is currently available about the capability of the different antigenic sites of gD to provide protective immunity upon vac- cination. Eisenberg et al. [72] showed that four immunizations with synthetic peptides 1-23 and 8-23 protected mice against a lethal dose of HSV- 2. Watari et al. [54] showed in addition, that one single dose of peptide 1-23 in another adjuvant formulation, was sufficient for protection. Geer- ligs et al. [29] used a chimeric protein containing residues 5-55 of gD to protect mice. Detailed analysis demonstrated that resistance could be obtained after immunization with peptide 9-21, but not with peptides 1-13, 18-30, 22-38 and 38-56. Besides the peptides from the N-terminal region, peptide 267-281 of the C-terminal region was investigated; it did not confer resistance upon vaccination (H.J. Geerligs, unpublished results). In addition, a chimeric protein containing residues
66
53-312 of gD could provide protect ion after im- munizat ion of mice [73]. It therefore seems that in animal model systems not only complete gD, but also several smaller regions of gD can provide protect ion against lethal challenge with HSV.
Studies on passive immunizat ion with m A b s against gB, gC, gD, gG, and gH revealed that depending on the properties of mAbs a certain degree of protect ion could be obtained. Dix et al. [74] found that passive immunizat ion with an anti- gD mAb of group Ia protected mice. In addition, Simmons et al. [75] showed that the spread of the infection could be limited by another group Ia m A b as well as by an unclassified one. Kohl et al. [58], using a murine model to s tudy neonatal HSV infections, demonst ra ted that protect ion against challence with a high viral dose only could be achieved by transfer of a combinat ion of ant ibod- ies against synthetic peptides of gD and exoge- nous leukocytes. Most effective in this combina- tion were the antibodies directed against peptides 2-12 , 52-61, 267-276, 288-297 and 302-312, which mediated an A D C C . Using a low viral dose, resistance was observed after transfer of anti- peptide antibodies, which are able to neutralize virus infectivity in vitro, e.g., antibodies against peptides 12-21, 288-297, 303-312 and 314-323. This clearly demonstrates the complexity of the mechanism by which protect ion against HSV in- fections may be obtained.
6. C O N C L U S I O N S
Glycoprotein D of HSV is one of the major antigenic components of the envelope of mature virions and is therefore a promising candidate for a HSV vaccine. The antigenic structure of a pro- tein is dependent on its primary, secondary and tertiary structure and it may vary depending on the origin of the elements of the immune system that interact with this structure. The determina- tion of the antigenic structure requires the avail- ability of native gD, fragments thereof (obtained either by proteolytic digestion, r e c o m b i n a n t - D N A methods, or organic synthesis) or muta ted forms of the protein. T cell epitopes are cont inuous and determinat ion of their localization on gD is now
in progress. There appear to be several T cell specific sites on gD with lack of immunodomi- nance in the response. Much progress has been made with respect to mapping of cont inuous B cell epitopes since the preparat ion of synthetic fragments is relatively easy. The sites reactive with polyclonal and mAbs almost completely cover the gD molecule. Discont inuous B cell epitopes are difficult to elucidate since they only exist in an intact conformat ion. Only the three-dimensional structure determinat ion of gD, complexed with antibodies, will allow an accurate assessment of the contacts between HSV-gD and antibodies di- rected to it.
R E F E R E N C E S
[1] Wildy, P. (1986) lntervirology 25, 117-140. [2] McGeoch, D.J., Dolan, A., Donald, S. and Rixon, F.J.
(1985) J. Mol. Biol. 181, 1-13. [3] McGeoch, D.J., Dalrymple, M.A., Davison, A.J., Dolan,
A., Frame, M.C., McNab, D., Perry, L.J., Scott, J.E. and Taylor, P. (1988) J. Gen. Virol. 69, 1531-1574.
[4] Ligas, M.W. and Johnson, D.C. (1988) J. Virol. 62, 1486- 1494.
[5] Highlander, S.L., Sutherland, S.L., Gage, P.J., Johnson, D.C., Levine, M. and Glorioso, J.C. (1987) J. Virol. 61, 3356-3364.
[6] Johnson, D.C. and Spear, P.G. (1989) J. Virol. 63, 819-827. [7] Minson, A.C., Hodgman, T.C., Digard, P., Hancock, D.C.,
Bell, S.E. and Buckmaster, E.A. (1986) J. Gen. Virol. 67, 1001-1013.
[8] Watson, R.J. (1983) Gene 26, 307-312. [9] Lasky, L.A. and Dowbenko, D.J. (1984) DNA 3, 23-29.
[10] Wilcox, W.C., Long, D., Sodora, D.L., Eisenberg, R.J. and Cohen, G.R. (1988) J. Virol. 62, 1941-1947.
[11] McGeoch, D.J. (1990) J. Gen. Virol. 71, 2361-2367. [12] Matthews, J.T., Cohen, G.R. and Eisenberg, R.J. (1983)
48, 521-533. [13] Serafini-Cessi, F., Dall'Olio, F., Pereira, L. and Campa-
delli-Fiume, G. (1988) J. Gen. Virol. 69, 869-877. [14] An'fit, A.G., Mariuzza, R.A., Philips, S.E.V. and Poljak,
R.J. (1986) Science 233, 747-753. [15] Sheriff, S., Silverton, E.W., Padian, E.A., Cohen, G.H.,
Smith-Gill, S.J., Finzel, J.B. and Davies, D.R. (1989) Proc. Natl. Acad. Sci. U.S.A. 84, 8075-8079.
[16] Tulip, W.R., Varghese, J.N., Webster, R.G., Air, G.M., Laver, W.G. and Colman, P.M. (1990) Cold Spring Harbor Symp. Quant. Biol. 54, 257-263.
[17] Shinnick, T.M., Sutcliffe, J.G., Green, N. and Lerner, R.A. (1983) Annu. Rev. Microbiol. 37, 425-446.
[18] Brown, F. (1984) Annu. Rev. Microbiol. 38, 221-235.
[19] Cwirla, S.E., Peters, E.A., Barrett, R.W. and Dower, W.J. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 6378-6382.
[20] Devlin, J.J., Panganiban, L.C. and Devlin, P.E. (1990) Science 249, 404-406.
[21] Scott, J.K. and Smith, G.P. (1990) Science 249, 386-389. [22] Laver, W.G., Air, G.M., Webster, R.G. and Smith-Gill,
S.J. (1990) Cell 61,553-556. [23] Bosch, D.L., Geerligs, H.J., Weijer,' W.J., Feijlbrief, M.,
Welling, G.W. and Welling-Wester, S. (1987) J. Virol. 61, 3607-3611.
[24] Cohen, G.H., Dietzschold, B., Ponce de Leon, M., Long, D., Golub, E., Varrichio, A., Pereira, L. and Eisenberg, R.J. (1984) J. Virol. 49, 102-108.
[25] Eisenberg, R.J., Long, D., Ponce de Leon, M., Matthews, J.T., Spear, P.G., Gibson, M., Lasky, L.A., Berman, P., Golub, E. and Cohen, G.H. (1985) J. Virol. 53, 634-644.
[26] Muggeridge, M., Roberts, S.R., Isola, V.J., Cohen, G.H. and Eisenberg, R.J. (1990) in Immunochemistry of Viruses. II. The Basis for Serodiagnosis and Vaccines (van Re- genmortel, M.H.V. and Neurath, A.R., Eds.), Elsevier Publishers B.V. (Biomedical Division) Amsterdam.
[27] Isola, V.J., Eisenberg, R.J., Siebert, G.R., Heilman, C.J., Wilcox, W.C. and Cohen, G.H. (1989) J. Virol. 63, 2325- 2334.
[28] Strynadka, N.C.J., Redmond, M.J., Parker, R.J.M., Scraba, D.G. and Hodges, R.S. (1988) J. Virol. 63, 3474-3483.
[29] Geerligs, H.J., Kocken, C.H.M., Drijfhout, J.W., Weijer, W.J., Bloemhoff, W., Wilterdink, J.B., Welling, G.W. and Welling-Wester, S. (1990) J. Gen. Virol. 71, 1767-1774.
[30] Geerligs, H.J., Feijlbrief, M., Bolk, M., Bos, C.A., Drijfhout, J.W., Welling, G.W. and Welling-Wester, S. (1990) Arch. Virol. 114, 251-258.
[31] Barker, W.C., Hunt, L.T., Orcutt, B.C., George, D.G., Yeh, L.S., Chen, H.R., Blomquist, M.C., Johnson, G.C., Seibel-Ross, E.I., Ledley, R.S. (1989) in Atlas of Protein Sequence and Structure-Protein Sequence Database, Ver- sion 1989, Natl. Biomed. Res. Foundation Washington, DC.
[32] Dayhoff, M.O., Barker, W.C. and Hunt, L.T. (1983) Methods Enzymol. 91, 524-545.
[33] Eisenberg, R.J., Long, D., Pereira, L., Hampar, B., Zweig, M., and Cohen, G.H. (1982) J. Virol. 41,478-488.
[34] Thomas, D.B., Burr, D.S., Barnett, B.C., Graham, C.M. and Skehel, J.J. (1990) Cold Spring Harbor Symp. Quant. Biol. 54, 487-495.
[35] Air, G.M., Laver, W.G., Webster, R.G., Els, M.C. and Luo, M. (1990) Cold Spring Harbor Symp. Quant. Biol. 54, 247-255.
[36] Cohen, G.H., Isola, V.J., Kuhns, J., Berman, P.W. and Eisenberg, R.J. (1986) J. Virol. 60, 157-166.
[37] Muggeridge, M.I., Isola, V.J., Byrn, R.A., Tucker, T.J., Minson, A. C., Glorioso, J.C., Eisenberg, R.J. and Cohen, G.H. (1988) J. Virol. 62, 3274-3280.
[38] Muggeridge, M.I., Wu, T.-T, Johnson, D.C., Glorioso, J.C., Eisenberg, R.J. and Cohen, G.H. (1990) Virology 174, 375-382.
67
[39] Rawls, W.E., Balachandran, N., Sissons, G. and Watson, D.H. (1984) J. Virol. 51, 263-265.
[40] Nash, A.A., Leung, K.-N. and Wildy, P. (1985) in The Herpesviruses, Vol. 4 (Roizman, B. and Lopez, C., Eds.), pp. 87-102, Plenum, New York.
[41] Zarling, J.M., Moran, P.M., Lasky, L.A. and Moss, B. (1986) J. Virol. 59, 506-509.
[42] DeFreitas, E.C., Dietzschold, B. and Koprowski, H. (1985) Proc. Natl. Acad. Sci. U.S.A. 82, 3425-3429.
[43] Rosenthal, K.L., Smiley, J.R., South, S. and Johnson, D.C. (1987) J. Virol. 61, 2438-2447.
[44] Martin, S., Moss, B., Berman, P.W., Lasky, L.A. and Rouse, B.T. (1987) J. Virol. 61,726-734.
[45] Blacklaws, B.A., Nash, A.A. and Darby, G. (1987) J. Gen. Virol. 68, 1103-1114.
[46] McLaughlin-Taylor, E., Willey, D.E., Cantin, D.M., Eberle, R., Moss, B. and Openshaw, H. (1988) J. Gen. Virol. 69, 1731-1734.
[47] Schrier, R.D., Pizer, L.I. and Moorhead, J.W. (1983) J. Immunol. 130, 1413-1417.
[48] Johnson, R.M., Lancki, D.W., Fitch, F.W. and Spear, P.G. (1990) J. Immunol. 145, 702-710.
[49] Witmer, L.A., Rosenthal, K.L., Graham, F.L., Friedman, H.M., Yee, A. and Johnson, D.C. (1990) J. Gen. Virol. 71, 387-396.
[50] Krishna, S., Blacklaws, B.A., Overton, H.A., Bishop, D.H.L. and Nash, A.A. (1989) J. Gen. Virol. 70, 1805- 1814.
[51] McDermott, M.R., Goldsmith, C.H., Rosenthal, K.R. and Brais, L.J. (1989) J. Infect. Dis. 159, 460-466.
[52] Rothbard, J.B. (1986) Ann. Inst. Pasteur 137E, 518-528. [53] Heber-Katz, E., Hollosi, M., Dietzschold, B., Hudecz, F.
and Fasman, G.D. (1985) J. Immunol. 135, 1385-1390. [54] Watari, E., Dietzschold, B., Szokan, G. and Heber-Katz,
E. (1987) J. Exp. Med. 165,459-470. [55] Yamashita, K. and Heber-Katz, E. (1989) J. Exp. Med.
170, 997-1002. [56] Wyckoff, J.H., Osmand, A.P., Eisenberg, R.J., Cohen,
G.H. and Rouse, B.T. (1988) Immunobiol. 177, 134-148. [57] Sprecher, E. and Becker, Y. (1987) J. Virol. 61, 2515-2522. [58] Kohl, S., West, M.S., Prober, C.G., Sullender, W.M. and
Arvin, A.M. (1989) J. Infect. Dis. 160, 770-776. [59] Kohl, S., Strynadka, N.C.J., Hodges, R.S. and Pereira, L.
(1990) J. Clin. Invest. 86, 273-278. [60] McKendall, R.R. (1977) Infect. Immun. 16, 717-719. [61] Wilson, L.A., Karabin, J.M., Smith, J.W., Dawson, D. and
Scott, D.W. (1984) J. Immunol. 132, 1522-1528. [62] Kuo, Y-C. and Lin, C-Y. (1990) J. Med. Virol. 31,183-189. [63] Weinberg, A., Konrad, M. and Merigan, T.C. (1987) J.
Virol. 61, 2120-2127. [64] Gebhardt, B.M. and Hill, J.M. (1988) J. Neuroimmunol.
28, 227-236. [65] Bohus, B. and Koolhaas, J.M. (1990) in Psychoneuroim-
munology, 2nd edn. (Ader, R., Cohen, N. and Felten, D., Eds.), pp. 807-830, Academic Press, England.
[66] Hall, M.J. and Katrak, K. (1986) Vaccine 4, 138-150.
68
[67] Chan, W.L. (1983) Immunology 49, 343-348. [68] Long, D., Madara, T.J., Ponce de Leon, M., Cohen, G.H.,
Montgomery, P.C. and Eisenberg, R.J. (1984) Infect. Im- mun. 37, 761-764.
[69] Inoue, Y., Ohashi, Y., Shimomura, Y., Manabe, R., Yamada, M., Ueda, S. and Kato, S. (1990) Invest. Oph- thalmol. Vis. Sci. 31,411-418.
[70] Stanberry, L.R., Bernstein, D.I., Burke, R.L., Pachl, C. and Myers, M.G. (1987) J. Infect. Dis. 155, 914-920.
[71] Wachsman, M., Aurelian, L., Smith, C.C., Perkus, M.E. and Paoletti, E. (1989) J. Infect. Dis. 159, 625-634.
[72] Eisenberg, R.J., Cerini, C.P., Heilman, C.J., Joseph, A.D.. Dietzschold, B., Golub, E., Long, D., Ponce de Leon, M. and Cohen, G.H. (1985) J. Virol. 56, 1014-1017.
[73] Broker, M., Abel, K.-J., Hilfenhaus, J. and Amann. E. (1990) Med. Microbiol. Immunol. 179, 145-159.
[74] Dix, R.D., Pereira, L. and Baringer, J.R. (1981) Infect. Immun. 134, 192-199.
[75] Simmons, A. and Nash, A.A. (1985) J. Virot. 53, 944-948.