Baruch Et Al 2000
Transcript of Baruch Et Al 2000
-
7/28/2019 Baruch Et Al 2000
1/13
Responses to Light and Water Availability of Four Invasive Melastomataceae in the HawaiianIslandsAuthor(s): Zdravko Baruch, Robert R. Pattison, Guillermo GoldsteinReviewed work(s):Source: International Journal of Plant Sciences, Vol. 161, No. 1 (January 2000), pp. 107-118Published by: The University of Chicago PressStable URL: http://www.jstor.org/stable/10.1086/314233 .
Accessed: 03/04/2012 12:00
Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at .http://www.jstor.org/page/info/about/policies/terms.jsp
JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of
content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms
of scholarship. For more information about JSTOR, please contact [email protected].
The University of Chicago Press is collaborating with JSTOR to digitize, preserve and extend access to
International Journal of Plant Sciences.
http://www.jstor.org/action/showPublisher?publisherCode=ucpresshttp://www.jstor.org/stable/10.1086/314233?origin=JSTOR-pdfhttp://www.jstor.org/page/info/about/policies/terms.jsphttp://www.jstor.org/page/info/about/policies/terms.jsphttp://www.jstor.org/stable/10.1086/314233?origin=JSTOR-pdfhttp://www.jstor.org/action/showPublisher?publisherCode=ucpress -
7/28/2019 Baruch Et Al 2000
2/13
107
Int. J. Plant Sci. 161(1):107118. 2000. 2000 by The University of Chicago. All rights reserved.1058-5893/2000/16101-0011$03.00
RESPONSES TO LIGHT AND WATER AVAILABILITY OF FOUR INVASIVEMELASTOMATACEAE IN THE HAWAIIAN ISLANDS
Zdravko Baruch,1,* Robert R. Pattison,2, and Guillermo Goldstein
*Deptartamento de Estudios Ambientales, Universidad Simon Bolvar, Apartado 89000, Caracas 1080, Venezuela; and Department of Botany,University of Hawaii, 3190 Maile Way, Honolulu, Hawaii 96822, U.S.A.
Plant invasion by Neotropical Melastomataceae is prominent in Hawaii. To understand life history traitsof four successful invasive Melastomataceae, two shade-intolerant herbs (Arthrostema ciliatum and Tibouchinaherbacea) and two shade-tolerant woody species (Clidemia hirta, a shrub, and Miconia calvescens, a tree)were subjected to three light levels and two watering regimes in a greenhouse. Plant height, leaf number andarea, biomass allocation, relative growth rate (RGR), carbon assimilation (A), leaf nutrient content, leafconstruction costs (CC), specific leaf mass (SLM), and leaf spectral properties were determined at the end ofthe experimental period. Plant size, total biomass, RGR, A, CC, and SLM decreased, whereas leaf lighttransmittance and leaf N increased under low light in all species. The effects of water stress were weaker than
light-stress effects. Relative growth rate of herbs grown in sun and partial shade (0.046 and 0.033 g g
1
d
1
,respectively) was higher than in the woody species (0.027 and 0.020 g g1 d1). Woody species allocated morebiomass to leaf production than herbs, which allocated more biomass to stem production. Shade increasedallocation of biomass to leaves, and water stress increased the root-shoot ratio in all species. Partial shadeincreased leaf area ratios more in the herbs (140%) than in woody species (68%). Miconia calvescens and C.hirta had higher leaf absorbance (92%) than both herbs (79%). Maximum A under all light treatments wassimilar in all species, and there was substantial acclimation to the different light levels. Leaf construction costwas higher in the apparently long-lived leaves of the woody species. Relative growth rate, carbon allocation,and SLM showed larger changes to light and water stress than A and related photosynthetic parameters. Allspecies showed responses qualitatively similar to those of other tropical species including the high acclimationpotential to light, but the herbs exhibited the largest quantitative responses. When compared with a largegroup of native species, the four melastomes appear to be better suited to capture and use light, which isconsistent with their rapid spread in mesic and disturbed Hawaiian environments.
Keywords: carbon assimilation, construction costs, leaf nutrients, melastomataceae, nitrogen use efficiency,plant growth, plant invasions, specific leaf mass, ecophysiology.
Introduction
Several species of the Neotropical family Melastomataceaeare among the most aggressive invaders of the Hawaiian andother Pacific islands that are particularly susceptible to plantinvasions (Vitousek et al. 1987; Loope and Mueller-Dombois1989; Smith 1992; Meyer and Florence 1996; Medeiros et al.1997). Introduced accidentally or as ornamentals, these specieshave displaced native plants throughout the islands followingdisturbances such as deforestation, fire, and overgrazing. In
addition to reproductive traits such as small seed size and highdispersion capability (Rejmanek and Richardson 1996), highgrowth rate, large carbon assimilation, and acclimation ca-pacity (Baruch et al. 1985; Bazzaz 1986; Williams and Black1994; Pattison et al. 1998; Baruch and Goldstein 1999) alsocontribute to the success of an invader plant. Apart from al-
1 Author for correspondence; fax 58-2-9063064; e-mail [email protected].
2 Current address: Department of Botany, Washington State Uni-versity, Pullman, Washington 99164-4238, U.S.A.
Manuscript received April 1999; revised manuscript received September 1999.
tering species composition and the structure and diversity ofisland communities, these invasions may also alter ecosystemfunction (Vitousek 1994).
Light and water are the most limiting resources for plantlife, as they are essential for the acquisition of carbon andmineral nutrients. Plant species usually differ in their adap-tations to the low availability of these two limiting resources.For example, shade-tolerant species benefit from adaptationsthat maximize light capture at low light levels and minimizerespiratory costs (Pearcy and Sims 1994). Lowlightavailability
generally increases the proportion of biomass allocated toleaves in order to compensate for reduced carbon fixation(Schulze and Chapin 1987). Shade-intolerant plants face water,nutrient, and photoinhibition stresses that can adversely in-teract with the maintenance of high photosynthetic rates(Pearcy and Sims 1994). In contrast, water stress increases theroot-shoot ratio and would tend to increase root surface areafor nutrient uptake (Chapin 1991). It is also possible that con-flicting situations occur where both light and water shortagestake place simultaneously. Growth and biomass allocation re-sponses to resource shortage are specific for each life-form andact as balancing mechanisms to maintain equilibrium in the
-
7/28/2019 Baruch Et Al 2000
3/13
108 INTERNATIONAL JOURNAL OF PLANT SCIENCES
internal budget of carbon and nutrients (Orians and Solbrig1977; Schulze 1981; Schulze and Chapin 1987).
Growth, carbon assimilation, and allocation and nutrientdynamics responses of tropical plants to light and to droughtstress have been studied in some detail in relation to habitat(open or forest gap vs. understory) and to succesional dynam-
ics. Some findings support that shade results in an increase ofplant height and specific leaf mass and decrease of growth rate(Bjorkman 1981; Chazdon and Field 1987; Fetcher et al. 1987;Denslow et al. 1990; Thompson et al. 1992; Ellison et al. 1993;Kitajima 1996), leaf number and leaf construction costs de-crease under shaded conditions (Williams et al. 1989; Denslowet al. 1990; Poorter and Villar 1997), and assimilation rate ona leaf area basis decreases and large acclimation capacity char-acterizes plants subjected to light limitation (Denslow et al.1990; Newell et al. 1993; Ellsworth and Reich 1996; Strauss-Debenedetti and Bazzaz 1996). A compilation of these studieshas been recently published (Mulkey et al. 1996). Althoughseveral melastomes have been included, specially by Ellison etal. (1993), none of the species studied here has been discussed.
From the 15 invading melastomes described for Hawaii (Al-meda 1990), we selected four species from different habitatsand with different growth forms as the most aggressive in-vaders: the forest tree Miconia calvescens DC, the shrub Cli-demia hirta L. D. Don, and the herbs from open sites, Ar-throstema ciliatum Pav. Ex D. Don. and Tibouchina herbacea(DC) Cogn. Here we attempt to answer the question How dolight and water availability, representative of the contrastingenvironments where these invading species colonize and per-sist, affect growth, carbon assimilation, and biomass alloca-tion? On the basis of their distribution in the Hawaiian Islands,we predict that the herbs from open sites will be most sensitiveto shade, whereas the forest understory seedlings of the treewill be better able to acclimate to different light regimes. The
shrub, which grows in both open and relatively shaded hab-itats, should exhibit intermediate responses to light. In addi-tion, we will compare our results with those of Pattison et al.(1998) and Baruch and Goldstein (1999) for native speciesthat may provide new insights to understand the success ofthe invasive melastomes in Hawaii.
Material and Methods
Species and Habitat
Miconia calvescens is a tree (up to 15 m tall) that was in-troduced as an ornamental in the Pacific islands. In Hawaii,it became one of the most serious threats in sites of up to 500
m elevation with more than 1800 mm of rainfall (Medeiroset al. 1997; Meyer and Malet 1997). This tree has large deepgreen leaves with a purple underside and produces large num-bers of seedlings in the understory of invaded rain forests(Meyer and Malet 1997). Its growth is enhanced when lightbecomes more available after the removal of parent trees fromthe canopy (Meyer and Malet 1997), indicating that M. cal-vescens seedlings may be shade tolerant and relatively droughtsensitive. Clidemia hirta is a small to medium-height (0.53m tall) shrub that in Hawaii occurs up to 1250 m in disturbedmesic sites and behaves as a typical secondary succession pi-oneer (Smith 1992; Medeiros et al. 1997). Because of its ca-
pacity to invade open habitats and persist under early stagesof forest succession, we consider it as intermediate in terms oflight requirements. This shrub shows leaf senescence, cessationof growth, and death of shoot tips during rainless periods(Smith 1992), indicating that it is also a drought-sensitive spe-cies. Arthrostema ciliatum is an annual or biannual herb, and
Tibouchina herbacea is a perennial herb. Both are up to 1.5m tall and found in open, disturbed, or ruderal sites (Wester1992; Medeiros et al. 1997). On the basis of their habitat,both herbs are probably shade-intolerant but relativelydrought-tolerant species. For convenience, these species willhereafter be cited only by genus.
Seed Source and Experimental Treatments
Miconia and Tibouchina seedlings were collected on the is-land of Maui, the former from a lowland secondary rain forestnear Hana and the latter from an open site along the roadfrom Kahului to Hana. Clidemia seedlings were collected atthe Lyon Arboretum in Honolulu (Oahu Island), and Arthros-
tema seedlings were grown from seeds collected from manyplants in the same area. Care was taken to collect seedlingswith intact roots, and they were promptly transplantedto smallpots. All species were grown for 1 mo under one layer of shadecloth (25% of full sunlight) in the greenhouse. Before thetreatments, all plants were transplanted into 4-L pots on a2 : 1 soil (Supersoil Potting Mix, San Francisco) to perlite mix,except those ofArthrostema, which were planted in 2.5-L potsbecause of their initial smaller size. All plants were fertilizedwith a N, P, and K controlled-release mixture (14 : 14 : 14) ata dose of 5.5 g (4 L) and 2.2 g (2.5 L). After 1 mo, fiveindividuals of each species were randomly selected and har-vested to determine plant height and leaf number and area andthe dry weight of leaves, stems, and roots in order to obtain
the initial data for relative growth rate calculation. Leaf areawas measured with an LI-3000A leaf area meter (LiCor, Lin-coln, Nebr.). Twenty individuals of each species were randomlyassigned to each of the following treatments: high light (fullsunlight in the greenhouse; HL), medium light (25% of fullsunlight; ML), and low light (4%5% of full sunlight; LL).Light levels were obtained by covering the greenhouse bencheswith none, one, or two layers of neutral density filters (shadecloths).
Each light treatment was subdivided into two water treat-ments, each with 10 individuals per species. Watered plantsreceived water every 23 d. Water was withheld for thedrought-stress plants until the first symptoms of wilting wereevident. Then they were watered to field capacity. All stressed
plants had at least two drying cycles. Every 2 wk, leaf numberswere counted, plant height was measured up to the top leaf,and phenology were determined in all plants. At midexperi-ment, the plants were randomly rotated under all treatments.The experimental period was 76 d for Arthrostema, 79 d forTibouchina, 81 d for Clidemia, and 85 d for Miconia in orderto allow for time for gas exchange measurements and to pro-cess the samples.
Environmental and Physiological Measurements
Air temperature and relative humidity in the greenhousewere monitored with Vaisala sensors (Helsinki). Leaf temper-
-
7/28/2019 Baruch Et Al 2000
4/13
BARUCH ET AL.LIFE HISTORY OF INVASIVE MELASTOMES 109
ature was measured with thermocouples attached to their ab-axial surface. Photosynthetic photon flux density (PPFD) wasmeasured with Li-Cor quantum sensors. All data were re-corded and averaged at 10-min intervals with a Campbell CR-10 data logger (Campbell Scientific, Logan, Utah). During thelast third of the experiment, carbon assimilation (A) and sto-
matal conductance (gs) were measured with a Li-Cor 6200 gasexchange system on young fully expanded leaves of four ran-domly selected individuals per species and pertreatment duringthe mornings of sunny days under the different growing lightconditions. Before the final harvest, at the end of the last dryingcycle, predawn leaf water potential (W) was measured on leafsegments of three randomly selected individuals per speciesand per treatment with a thermocouple psychrometer (ModelSC-10, Decagon Devices, Pullman, Wash.). The percentage ra-diation reflected (r) and transmitted (t) by young fully ex-panded leaves of three randomly selected individuals per spe-cies of the watered plants was measured on their adaxialsurface with a Li-Cor 1800-12S integrating sphere connectedto a quantum sensor. The percentage of radiation absorbed by
the leaf (a) was calculated as . Carbon assim-a = 100 (r t)ilation (A) and gs were measured on the youngest fully ex-panded leaf of three watered individuals per treatment perspecies. After an initial dark respiration measurement, PPFDwas increased stepwise to 1500 mmol m2 s1. Under eachPPFD level, the leaf was allowed to equilibrate for 30 minbefore measurement. Light was generated with a QBEAM2001 lamp (Quantum Devices, Barneweld, Wis.). Quantumyield, light saturation, and compensation points were calcu-lated from the relationship between A and PPFD, corrected bythe leaf absorbance of each species. On completion of lightresponse curves, leaf disks were taken for specific leaf mass(SLM) determination.
Growth Analysis, Nutrient and EnergyConcentration, and Statistics
At harvest, plant height, leaf number and area, and biomassof all plants were measured as indicated above. Relativegrowth rate was calculated per plant and per leaf number andarea, plant height, and total biomass were calculated as
, where Wis both the initial and finalRGR = ln W ln W/t tf i f ivalues of the variable measured, and the denominator is thetime elapsed between the initial and final measurements. Leafarea ratio (LAR) was calculated as the relationship betweenleaf area and total plant mass. Leaf N, P, and K concentrationswere determined by micro-Kjeldahl (Nelson and Sommers1972) and inductive plasma spectrometry methods (Isaac and
Johnson 1983). An aliquot of dried leaves was employed forthe determination of energy concentration of the tissues on anash-free dry weight basis with a bomb calorimeter (Parr In-struments, Moline, Ill.). Leaf construction costs (CC) were cal-culated from N (considering ammonium as the source of N),energy, and ash concentrations (Williams et al. 1987). Instan-taneous photosynthetic nitrogen use efficiency (PNUE) was cal-culated as A/[N], and the rate of photosynthesis by unit leafCC was calculated as A/CC. In both cases, the A correspondsto that measured under the maximum PPFD.
Statistical analysis was performed separately for each specieswith a two-way ANOVA with light and water treatments as
the main factors. When applicable, a one-way ANOVA wasapplied for differences among species. A Tukey test was usedto analyze the differences between the means of the treatments(SYSTAT 1997). The relationships between RGR and othervariables were analyzed by multiple regression.
Results
Growth Conditions, Mortality, and Phenology
Mean maximum and minimum air temperature and relativehumidity for the treatments were 34.4 and 19.8C and 85.1%and 39.1%, respectively. Mean daylight maximum leaf tem-peratures were 32.8 and 29.8C for HL and LL conditions,respectively. Mean maximum SE PPFDs were ,783 163
, and mmol m2 s1 in the HL, ML, and LL208 48 34 6treatments, respectively. The lowest predawn Ws attained dur-ing water stress ranged from 0.67 MPa in Tibouchina to0.82 MPa in Arthrostema. The predawnWs ofClidemia and
Miconia minima were intermediate to those of the two herbs.The watered control plants ranged between 0.25 and 0.29MPa for all species. All species except Clidemia exhibited somemortality under the LL treatment. The highest mortality was60% and 70% for Tibouchina under the watered and water-stressed treatments, respectively. Water-stressed Miconia andArthrostema had 20% and 10% mortality, respectively. Ar-throstema flowered after 60 d from the beginning of the ex-periment but only under the HL and ML watered treatments.The biomass of reproductive parts was !1% of the total plantbiomass.
Plant Size, Leaf Number, Leaf Area,
and Leaf Specific MassExcept under LL,watered plants were the tallest in allspecies
(table 1). Water stress affected the height of Arthrostema themost, reducing it to 25% of the watered controls. Except inwatered Arthrostema and Miconia, ML plants were the tallest,and LL plants were the smallest. Under both watering treat-ments, plant height of the herbs Arthrostema and Tibouchinashowed the largest responses to light (table 1). Watering in-creased leaf area and number in all species, and LL inducedthe production of fewer leaves with lower total leaf area perplant. Thelargest leaf area under drought stress occurred underML (table 1). The largest leaf area in watered Arthrostemaand Clidemia was also observed under ML. The effects ofwater and light regimes on leaf number and total leaf area
were much more pronounced in Arthrostema and Tibouchina,whose leaves were three to 11 times smaller than those ofClidemia and Miconia (table 1). Specific leaf mass was lowestin Arthrostema, which was unaffected by water stress. In theother species, drought induced the production of thicker leaves(table 1). Under all watering and light conditions, the woodyClidemia and Miconia had the highest SLM (table 1). All spe-cies produced leaves with significantly lower SLM under LL(table 1). Leaf senescence, although not measured specifically,was more pronounced in the herbs than in the woody species.Also, leaf production, measured as the increase of leaf numberbetween censuses, was higher in the herbs (data not shown).
-
7/28/2019 Baruch Et Al 2000
5/13
110 INTERNATIONAL JOURNAL OF PLANT SCIENCES
Table 1
Mean and Standard Error (in Parentheses) of Architecture-Related Traits under All Treatments
Treatments
Watered Water stressed ANOVA
Traits and species Highlight Mediumlight Lowlight Highlight Mediumlight Lowlight Water Light Interaction
Plant height (cm):Herbs:
Arthrostema . . . . . .. 29.6(3.6)
53.3(4.2)
26.2(1.6)
115.6(4.3)
89.3(2.1)
23.0(1.5)
* * *
Tibouchina . .. .. .. .. 44.8(5.6)
77.1(2.0)
32.0(5.5)
81.6(3.8)
87.3(1.3)
37.7(3.3)
* * *
Woody:Clidemia . .. .. .. .. .. . 30.4
(1.4)40.1(2.3)
31.1(2.0)
37.3(1.7)
49.1(2.0)
30.3(1.8)
* * *
Miconia . .. .. .. .. .. . 12.7(1.9)
13.7(1.2)
11.7(1.5)
18.7(1.3)
16.2(0.7)
11.1(0.5)
* * *
Leaf area (cm2):Herbs:
Arthrostema . .. .. . . 111.4(17.0)
411.2(35.4)
184.6(25.7)
986.9(60.2)
1144.0(71.0)
127.1(28.7)
* * *
Tibouchina . . .. .. . .. 455.2(44.6)
525.0(25.6)
45.7(19.9)
1810.7(126.6)
1339.7(214.8)
69.7(23.8)
* * *
Woody:Clidemia . . . .. . . .. . . . 724.0
(53.5)765.6(70.2)
411.9(33.4)
1139.0(81.8)
1287.8(112.8)
404.1(29.0)
* * *
Miconia . . . .. . . .. . . . 382.1(40.7)
562.7(75.7)
153.7(37.0)
1019.8(160.6)
822.6(105.7)
219.5(37.9)
* * *
Leaf number:Herbs:
Arthrostema . . . . . .. 20.8(2.1)
37.6(1.9)
20.5(2.2)
122.2(10.6)
90.8(6.8)
17.4(2.2)
* * *
Tibouchina . .. .. .. .. 62.2(5.9)
52.5(5.3)
11.6(1.3)
204.4(16.1)
98.1(16.0)
11.7(1.9)
* * *
Woody:Clidemia . .. .. .. .. .. . 35.7
(3.4)23.4(1.8)
14.6(0.8)
54.2(4.0)
32.5(1.7)
14.6(0.9)
* * *
Miconia . .. . . . .. . . . . 7.2(0.2)
7.2(0.3)
5.2(0.2)
10.5(1.2)
7.1(0.4)
5.8(0.2)
* * *
Specific leaf mass (g m2):Herbs:
Arthrostema . . . . . . . 29(1)
15(0.6)
9(0.1)
27(1)
16(1)
12(3)
ns * *
Tibouchina . . . . . . . . . 50(3)
26(1)
16(3)
37(2)
21(1)
18(5)
* * *
Woody:Clidemia . .. . . . .. . . . . 59
(1)36(1)
25(1)
49(2)
33(1)
20(1)
* * ns
Miconia . .. . . . .. . . . . 65(4)
42(1)
30(3)
60(1)
37(2)
24(2)
* * ns
Note. Values of (*) are statistically significant; significant.P ! 0.05 ns = not
Biomass Production, Allocation, andRelative Growth Rate
In all species and treatments, plant biomass decreased fromHL to LL. This was more pronounced in both herb species.Water-stressed Arthrostema and Miconia in HL and ML treat-ments had similar total plant biomass. Watered plants pro-duced more biomass than stressed plants. This difference wasmore evident under HL (table 2).
With the exception of Miconia, the root-shoot ratio de-
creased with decreasing light intensity in watered plants. In
drought-stressed plants, however, it decreased in Arthrostema
and Clidemia but increased in Miconia and Tibouchina as light
intensity decreased (table 2). In both cases, the changes in root-
shoot ratio in the woody Clidemia and Miconia were thesmall-
est. In all species, the root-shoot ratio increased under water
stress. Proportionally, Clidemia and Miconia allocated more
biomass to leaves than either of the two herbs, whereas Ar-
throstema and Tibouchina allocated a higher proportion to
-
7/28/2019 Baruch Et Al 2000
6/13
BARUCH ET AL.LIFE HISTORY OF INVASIVE MELASTOMES 111
Table 2
Mean and Standard Error (in Parentheses) of Total Biomass, Relative Growth Rate (RGR), and Leaf Area Ratio (LAR)
Treatments
Water stressed Watered ANOVA
Traits and species Highlight Mediumlight Lowlight Highlight Mediumlight Lowlight Water Light Interference
Total biomass (g):Herbs:
Arthrostema . . . . . . 1.39(0.20)
1.88(0.18)
.38(0.06)
12.84(0.97)
5.76(0.38)
0.24(0.04)
* * *
Tibouchina . .. .. .. 5.44(0.88)
3.49(0.21)
0.28(0.11)
20.63(1.56)
6.79(1.11)
0.31(0.06)
* * *
Woody:Clidemia . .. .. .. .. . 8.26
(0.61)5.30
(0.67)1.90
(0.18)10.52(1.04)
7.50(0.64)
1.45(0.16)
* * ns
Miconia . .. .. .. .. .. 4.32(0.58)
3.71(0.43)
0.96(0.12)
9.99(1.59)
4.21(0.47)
0.87(0.16)
* * ns
Root-shoot ratio:Herbs:
Arthrostema . . . . . . 0.72(0.04)
0.29(0.01)
0.25(0.02)
0.33(0.01)
0.18(0.01)
0.17(0.02)
* * *
Tibouchina . .. .. .. 0.41(0.03)
0.21(0.01)
0.67(0.18)
0.54(0.03)
0.14(0.01)
0.28(0.03)
* * *
Woody:Clidemia . .. .. .. .. . 0.47
(0.03)0.35
(0.03)0.37
(0.02)0.33
(0.02)0.24
(0.01)0.28
(0.02)* * ns
Miconia . .. .. .. .. .. 0.46(0.03)
0.33(0.02)
0.60(0.10)
0.34(0.01)
0.21(0.02)
0.34(0.04)
* * ns
RGR (g g1 d1):Herbs:
Arthrostema . .. .. . 0.0152(0.002)
0.0195(0.001)
0.0012(0.002)
0.0440(0.001)
0.0345(0.001)
0.0060(0.002)
* * *
Tibouchina . . .. .. . 0.0300(0.002)
0.0253(0.001)
0.0053(0.004)
0.0481(0.002)
0.0322(0.002)
0.0033(0.002)
ns * ns
Woody:Clidemia . . .. .. . .. . 0.0284
(0.001)0.0224
(0.001)0.0105
(0.001)0.0315
(0.001)0.0276
(0.001)0.0076
(0.001)ns * *
Miconia . .. . .. .. . .. 0.0146(0.001)
0.0129(0.001)
0.0023(0.002)
0.0244(0.002)
0.0149(0.001)
0.0043(0.002)
* * *
LAR (cm2 g1):Herbs:
Arthrostema . .. .. . 86.3(11.3)
223.0(8.8)
500.4(21.0)
77.7(2.5)
201.1(8.3)
541.5(72.6)
* ns ns
Tibouchina . .. .. .. 87.0(5.8)
152.7(8.9)
171.9(51.5)
88.5(3.9)
198.3(4.0)
203.8(45.7)
* ns ns
Woody:Clidemia . . .. .. . .. . 88.3
(3.1)151.7
(7.9)222.7(11.7)
111.6(5.0)
172.3(6.7)
293.1(21.4)
* * ns
Miconia . .. . .. .. . .. 93.7(8.5)
149.1(4.3)
156.1(9.0)
110.1(10.2)
193.7(17.2)
258.4(26.6)
* * ns
Note. Symbols as in table 1.
stems (data not shown). The young stems of both herbs aregreen and presumably capable of carbon assimilation. Withthe exception of Tibouchina, root biomass was highest in thedrought-stressed and HL treatment.
Herbs had higher RGRs in the watered high- and medium-light treatments. Watering increased RGR in all species butwas only statistically significant in Arthrostema and Miconia.
Light treatment had a significant affect on all species: plantsunder LL had the lowest and even negative RGR as determinedby changes in total biomass. Clidemia was the least affectedby LL (table 2). The negative RGRs under LL were causedmainly by decreases in stem and root biomass (data notshown). In all species, LAR increased significantly as light lev-els decreased. Watered plants had higher LARs than stressed
-
7/28/2019 Baruch Et Al 2000
7/13
112 INTERNATIONAL JOURNAL OF PLANT SCIENCES
plants (table 2). Interspecific differences in LAR were smallerunder HL and ML than under LL. Arthrostema had the highestLAR under LL (table 2).
Leaf Nutrients, Construction Costs, andSpectral Properties
Under any treatment, Arthrostema leaves had the highestleaf N concentration on a mass basis (table 3). Water-stressedplants of all species had significantly lower leaf N concentra-tion, whereas shade increased N content in all species exceptClidemia. On an area basis, leaf N was higher in the woodyplants because of their higher SLM. Leaf K responded similarlyto N, whereas leaf P concentration did not show any consistentpattern except that it decreased significantly as light decreasedin Clidemia (table 3). Leaf CC was lowest in Tibouchina andshowed a tendency to decrease as the intensity of the light leveldecreased (table 3). The two woody species and Arthrostemahad the highest leaf CC (table 3). Lack of replicates preventedfurther statistical analysis.
Except in Tibouchina and Miconia, leaf reflectance de-creased under shade (table 4). Leaf transmittance increasedsignificantly in all species, possibly because of the thinnerleaves developed under LL. Leaf absorbance decreased underLL in all species except Arthrostema (table 4). The light-demanding herbs Arthrostema and Tibouchina had the highestleaf reflectance and transmittance, whereas the relativelyshade-tolerant Miconia and Clidemia showed the highest leafabsorbances. The highest leaf absorbance was that ofMiconia,with their red-pigmented abaxial surface and high SLM.
Photosynthetic Gas Exchange
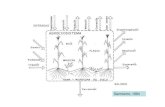
During gas exchange measurements, leaf temperature andrelative humidity in the cuvette ranged between 25 and 29C
and between 50% and 60%, respectively. Flowering of Ar-throstema under ML and HL probably induced changes incarbon metabolism that resulted in extremely low and unre-liable values of A (fig. 1). However, it was possible to extrap-olate A from previous measurements under HL in the green-house. Therefore, Arthrostema data are shown only forcomparison. Maximum photosynthesis on an area basis de-creased significantly in all species with decreasing PPFD grow-ing conditions (table 5). In contrast, because of the decreasein SLM, A on a mass basis showed no significant differencesamong treatments in any species (table 5). Arthrostema andTibouchina were the most efficient photosynthetic N users (ta-ble 5), and under HL, they were also the most efficient in fixingcarbon per unit leaf CC (table 4). Assimilation rate strongly
affected RGR of all species but more in herbs than in thewoody plants, as evidenced by a steeper slope of the re-gression between A and RGR in the former (herbs,
A; , ; woody2RGR =0.0053 0.0056 R = 0.90 P = 0.0001species A ; ; ).2RGR = 0.0015 0.0030 R = 0.69 P = 0.0007
In all species except Arthrostema, HLand MLplants showedsimilar light response curves, and the maximum A measureddecreased with decreasing PPFD growing conditions (fig. 1).In all species, light saturation took place in the range between400 and 1000 mmol m2 s1 for HL and ML treatments,whereas it occurred at 100200 mmol m2 s1 for LL plants(fig. 1). Quantum yield ranged between 0.053 and 0.114 mol
CO2 mol photons1 and increased significantly as the light
intensity of the treatment decreased. Dark respiration de-creased significantly from HL to lower light intensities only inClidemia but did not show any apparent trend in Miconia orTibouchina (table 5). Under HL, gs of both herbs was thehighest, but this difference disappeared under LL.In allspecies,
gs decreased as light regime decreased (table 4).
Discussion
Light and water availability and their interaction affectedmost of the measured variables, but the effects of water stresswere weaker than light-stress effects. The magnitude of theresponses was related to growth form. With few exceptions,the studied melastomes showed similar qualitative results toother tropical plants. Our hypothesis was confirmed, as theherbs from open habitats were most sensitive to light depri-vation, whereas the seedlings of Miconia were better able toacclimate to low light.
Light intensity of the treatments was similar to that foundin natural habitats such as large forest gaps, medium-sizedgaps, and the understory of forests (Denslow et al. 1990; Chaz-don et al. 1996). The range of temperatures was similar tomost mesic sites in the Hawaiian Islands. The high mortalityofTibouchina under LL indicates that this species may not beable to meet its metabolic requirements under deep shade,thereby restricting its habitat to open sites. The early floweringof Arthrostemas watered HL and ML plants indicates plas-ticity in phenology that may constitute a valuable trait for aninvader species (Bazzaz 1986). This flowering event may havetriggered senescence-related processes that deteriorated thephotosynthetic machinery, as evidenced by the low photosyn-thetic rates measured after flowering.
Plant Size, Leaf Number and Area, andSpecific Leaf Mass
All ML plants, except watered Arthrostema, were as tall oreven taller than HL plants. This response was not accompaniedby a parallel increase in plant mass, although the proportionof plant biomass allocated to stems did increase (data notshown), indicating that the stems developed under ML weremore slender and less compact. Plants under LL also showeda tendency to grow taller, particularly in the shade-tolerantClidemia and Miconia species. This behavior has been inter-preted as a response to enhance the capture of additional light,mediated by the phytochrome system and activated by a de-crease in the red : far red ratio under shade (Lee at al. 1996).
This was most likely not the case in this experiment since itwas performed under the shade provided by neutral densityfilters that do not alter the spectral composition of light. In-creased plant height under shade was also found in seedlingsof Heliocarpus appendiculatus, an early successional shade-intolerant tropical tree (Fetcher et al. 1987).
Plant leaf area of all species also increased under ML, whichresulted in the highest individual average leaf size in ML plants(data not shown). This response to light was more pronouncedin the herbs, which, as fast-growing species, could reallocatecarbon faster than the woody Clidemia and Miconia plants.For all species, SLM decreased as light availability decreased.
-
7/28/2019 Baruch Et Al 2000
8/13
BARUCH ET AL.LIFE HISTORY OF INVASIVE MELASTOMES 113
Table 3
Mean and Standard Error (in Parentheses) of Leaf Nutrients and Leaf Construction Costs
Treatments
Water stressed Watered ANOVA
Traits and species Highlight Mediumlight Lowlight Highlight Mediumlight Lowlight Water Light Interaction
Leaf N (%):Herbs:
Arthrostema . . .. . . .. . . .. . . .. . 1.92(0.26)
3.00(0.07)
2.98(0.08)
2.45(0.16)
3.02(0.14)
3.91)
* * ns
Tibouchina . . . .. . . .. . .. . . .. . . . 1.69(0.08)
2.52(0.01) NA
2.04(0.10)
2.85(0.11)
2.91)
) ) )
Woody:Clidemia . . .. . . .. . . .. . . .. . .. . . 1.34
(0.05)1.73
(0.08)1.87
(0.11)2.11
(0.08)2.10
(0.07)1.94
(0.03)* * *
Miconia . . . .. . . .. . . .. . .. . . .. . . 1.51(0.19)
1.76(0.01)
2.47(0.13)
1.85(0.19)
2.88(0.10)
2.53(0.11)
* * *
Leaf P (%):Herbs:
Arthrostema . . .. . . .. . . .. . .. . . 0.24(0.01)
0.28(0.02)
0.29(0.01)
0.32(0.02)
0.29(0.02)
.35)
ns ns ns
Tibouchina . . . .. . . .. . .. . . .. . . . 0.14(0.01)
0.20(0.01) NA
0.18(0.01)
0.21(0.01)
0.24)
) ) )
Woody:Clidemia . . .. . . .. . . .. . . .. . .. . . 0.50
(0.02)0.24
(0.01)0.27
(0.01)0.43
(0.02)0.28
(0.02)0.27
(0.02)ns * ns
Miconia . . . .. . . .. . . .. . .. . . .. . . 0.34(0.06)
0.24(0.01)
0.29(0.03)
0.32(0.06)
0.30(0.03)
0.42(0.03)
ns ns ns
Leaf K (%):Herbs:
Arthrostema . . .. . . .. . . .. . .. . . 3.43(0.18)
2.90(0.22)
5.44(0.27)
3.79(0.31)
2.94(0.42)
5.22)
ns * ns
Tibouchina . . . .. . . .. . .. . . .. . . . 0.51(0.03)
0.93(0.07) NA
0.69(0.10)
0.87(0.06)
0.75)
) ) )
Woody:Clidemia . . .. . . .. . . .. . . .. . .. . . 0.85
(0.09)1.18
(0.11)1.10
(0.12)1.26
(0.10)1.41
(0.09)1.29
(0.10)* ns ns
Miconia . . . .. . . .. . . .. . .. . . .. . . 0.84(0.11)
1.06(0.05)
1.54(0.13)
0.82(0.09)
1.39(0.02)
1.79(0.05)
* * ns
Construction costs (g glucose g1):Herbs:
Arthrostema . . . . . . . . . . . . . . . . . 1.27 1.21 NA 1.31 1.24 NA ) ) )Tibouchina . . . . . . . . . . . . . . . . . . . 1.18 1.18 NA 1.17 1.20 NA ) ) )
Woody:Clidemia . . . . . . . . . . . . . . . . . . . . . 1.27 1.25 1.22 1.29 1.24 1.22 ) ) )Miconia . . . . . . . . . . . . . . . . . . . . . . 1.26 1.23 NA 1.24 1.22 1.20 ) ) )
Note. Symbols as in table 1; not available. For the treatments in which there was not enough material for analysis, the replicatesNA = datawere pooled and do not show standard error or ANOVA.
This response to low light is common to melastomes (Denslowet al. 1990; Ellison et al. 1993) and other tropical plants(Bjorkman 1981; Chazdon and Field 1987; Fetcher et al. 1987;Thompson et al. 1992; Ellsworth and Reich 1996; Pattison etal. 1998). Less dense and thinner leaves may permit light toreach deeper chloroplasts in the leaf mesophyll, which is animportant adaptive trait in shaded habitats (Bjorkman 1981).The simultaneous increases in leaf area and decreases of SLMunder low light have been explained as a response to increasecarbon capture and to recover internal carbon balances (Bjork-man 1981; Schulze and Chapin 1987).
Drought stress significantly increased SLM in all species un-
der all light treatments. The production of thicker leaves underwater stress is a common response of plants to drought (War-ing 1991). In addition, thick leaves may increase the resistanceto water flow, thus reducing water loss and improving wateruse efficiency (Nobel 1991). The plasticity in SLM may allowa plant to regulate and to balance various internal processesand biomass allocation in response to environmental demands,acting as a homeostatic mechanism (Lambers and Poorter1992). Although the qualitative responses to water and lightstresses were similar in all species, the quantitative changes inplant height, leaf area, and SLM were largest in the herbs,indicating that these plants can respond faster to changes in
-
7/28/2019 Baruch Et Al 2000
9/13
114 INTERNATIONAL JOURNAL OF PLANT SCIENCES
Table 4
Mean and Standard Error (in Parentheses) of Maximum Photosynthesis Rate per Unit of Leaf Construction Costs(A
maxCC1) (in Equivalents of Glucose per Gram Tissue Dry Weight), Stomatal Conductance (g
s),
Leaf Transmittance, and Absorbance of Watered Plants
Traits and species
Treatments
PHigh light Medium light Low light
Amax CC1 (mmol CO2 g glucose
1 s1):Herbs:
Arthrostema . .. .. .. .. .. .. .. .. .. .. . 0.360(0.031)
NA NA )
Tibouchina . .. .. .. .. .. .. .. .. .. .. .. . 0.192(0.011)
0.335(0.047)
NA *
Woody:Clidemia . .. .. .. .. .. .. .. .. .. .. .. .. . 0.131
(0.023)0.165
(0.028)0.017
(0.015)ns
Miconia . .. .. .. .. .. .. .. .. .. .. .. .. .. 0.120(0.022)
0.111(0.022)
0.125(0.014)
ns
gs (mmol H20 m2 s1):
Herbs:Arthrostema . .. .. .. .. .. .. .. .. .. .. . 0.213
(0.092)NA 0.113
(0.023)*
Tibouchina . .. .. .. .. .. .. .. .. .. .. .. . 0.203(0.020)
0.275(0.035)
0.146(0.007)
*
Woody:Clidemia . .. .. .. .. .. .. .. .. .. .. .. .. . 0.163
(0.012)0.166
(0.019)0.142
(0.029)ns
Miconia . .. .. .. .. .. .. .. .. .. .. .. .. .. 0.190(0.020)
0.106(0.021)
0.105(0.021)
ns
Leaf transmittance (%):Herbs:
Arthrostema . .. .. .. .. .. .. .. .. .. .. . 10.11(1.22)
11.08(0.46)
15.43(0.27)
*
Tibouchina . .. .. .. .. .. .. .. .. .. .. .. . 9.01(1.04)
11.83(0.20)
16.17(1.45)
*
Woody:
Clidemia . .. .. .. .. .. .. .. .. .. .. .. .. . 3.27(0.52)
6.55(0.28)
11.26(0.83)
*
Miconia . .. .. .. .. .. .. .. .. .. .. .. .. .. 0.75(0.08)
0.96(0.13)
3.37(0.15)
*
Leaf absorbance (%):Herbs:
Arthrostema . .. .. .. .. .. .. .. .. .. .. . 77.69(1.85)
79.68(0.80)
77.30(0.44)
ns
Tibouchina . .. .. .. .. .. .. .. .. .. .. .. . 82.36(1.49)
78.85(0.14)
73.79(1.68)
*
Woody:Clidemia . .. .. .. .. .. .. .. .. .. .. .. .. . 88.24
(0.70)85.78(0.41)
81.16(0.88)
*
Miconia . .. .. .. .. .. .. .. .. .. .. .. .. .. 96.71(0.05)
97.11(0.09)
94.11(0.13)
*
Note. P indicates the significance of the one-way ANOVA on the difference between the light treatments. Symbolsas in table 1. Amax CC
1 and gs data for high-light Arthrostema are from greenhouse measurements.
environmental demands. Under similar full-light conditions,the melastomes had lower SLM than a large group of nativeHawaiian plants (melastomes: ; natives:39.5 15.0 81.9
g m2) (Pattison et al. 1998; Baruch and Goldstein 1999).41.7Low SLM appears to be a common trait of invader plants inHawaii as compared with native species and may contributeto their success as larger assimilatory surfaces are producedfor a given amount of carbon fixed.
Biomass Production, Allocation, andRelative Growth Rate
Shade and water stress decreased total biomass in all species.However, this decrease was proportionally lesser in the rela-tively slow-growing woody Miconia and Clidemia than in bothherbs. Under optimal growing conditions, herbs allocated pro-portionally more biomass to stems, whereas the woody species
-
7/28/2019 Baruch Et Al 2000
10/13
BARUCH ET AL.LIFE HISTORY OF INVASIVE MELASTOMES 115
Fig. 1 Light response curves of the plants grown under high light (open circles), medium light (triangles), and low light (filled circles). Barsindicate one standard error of the mean. Different letters for each light level indicate statistical significant means at . High- and medium-P ! 0.05light Arthrostemaplants displayed leaf senescence.
allocated more to leaves. In an open habitat, tall herbs havecompetitive advantage. In contrast, woody and relatively slow-growing plants would find no advantage by promoting stemgrowth, as they could not reach the canopy in the short term.For these species at the seedling stage, carbon allocation toleaves would be an available optimal avenue to increase re-source capture.
In response to water shortage, all species except Tibouchinaincreased the root-shoot ratio under HL and ML. This mayhelp to increase nutrient and water absorption when carbonincorporation is not limited (Schulze and Chapin 1987). Thecombined stresses of light and water deprivation lead to mor-tality, particularly in Tibouchina. Under light limitation, LAR
increased in all species, with Arthrostema showing the largestresponse. This was probably a consequence of a larger decreasein total plant biomass than in leaf area, resulting in an in-creased carbon incorporation that tended to balance the in-ternal carbon reserves (Schulze and Chapin 1987). A similarresponse to light limitation was found for a group of invasivespecies in Hawaii (Pattison et al. 1998). Carbon allocationchanges in response to water and light stresses were the largestin Arthrostema and the smallest in Clidemia. In this regard,the former showed the largest acclimation responses.
The RGR of both herbs was in the range of pioneer species(Kitajima 1996) and other invasive plants in Hawaii (Pattison
et al. 1998). Relative growth rates of the woody and shade-tolerant Miconia and Clidemia were substantially lower thanthose of the herbs but similar to those of other melastomes inCentral America (Denslow et al. 1990) and shade-tolerantplants in general (Kitajima 1996). The RGR of all species re-sponded strongly to both water and/or light stresses as ex-pected. In all species, RGR decreased significantly as the in-tensity of the growing light conditions decreased. The maincause of this decrease was the strongly correlated decrease ofA. A parallel decrease in SLM and the increase in LAR wasnot enough to counter the strong reduction in A that mighthave helped to maintain RGR levels. Clidemia was unique inmaintaining positive RGR even under a level of LL that, con-
sidering only RGR responses, would categorize it as the mostshade-tolerant species. Under similar conditions, the RGR ofthe four melastomes was 250% larger than that for a groupof native trees and herbs (melastomes, 0.036 g g1d1; natives,0.010 g g1d1) (Pattison et al. 1998). High RGR is one of themain traits that characterize invader species (Bazazz 1986),and it apparently contributes to the high invasive potential ofthe melastomes.
Leaf Nutrients, Spectral Properties, and Construction Cost
The significantly higher leaf Nmass in the plants under LLobserved in this experiment contrasts with observations on
-
7/28/2019 Baruch Et Al 2000
11/13
116 INTERNATIONAL JOURNAL OF PLANT SCIENCES
Table 5
Mean and Standard Error (in Parentheses) of MaximumPhotosynthesis Amax on Leaf Area and Weight Basis,
Photosynthetic Nitrogen Use Efficiency
(PNUE), and Dark Respirationof Watered Plants
Traits and species
Treatments
PHighlight
Mediumlight
Lowlight
Amax (mmol m2 s1):
Herbs:Arthrostema . . .. . . 10.63
(.77)NA 2.90
(.38)*
Tibouchina . .. .. .. 7.26(.81)
8.33(1.21)
2.85(.94)
*
Woody:Clidemia . . .. .. . .. . 8.29
(.29)6.14(.76)
3.59(.27)
*
Miconia . .. . .. .. . .. 7.72(1.40)
4.54(1.23)
2.89(.42)
*
Amax (mmol g1 s1):
Herbs:Arthrostema . .. .. . 0.482
(.051)NA 0.242
(.055)*
Tibouchina . . .. .. . 0.225(.013)
0.404(.057)
0.268(.076)
ns
Woody:Clidemia . . .. .. . .. . 0.169
(.027)0.206(.035)
0.208(.018)
ns
Miconia . . . .. . .. . . . 0.150(.031)
0.136(.027)
0.151(.017)
ns
PNUE (mmol gN1 s1):Herbs:
Arthrostema . . .. . . 19.59(1.01)
NA 6.19(1.40)
*
Tibouchina . . . .. . . 11.15(1.14)
14.17(2.04)
6.57)
ns
Woody:Clidemia . . .. .. . .. . 8.15
(1.60)9.95
(2.06)10.69
(.81)ns
Miconia . .. . .. .. . .. 8.19(1.61)
4.82(1.11)
5.99(.69)
ns
Dark respiration (mmolm2 s1):
Herbs:Arthrostema . . . . . . NA NA 0.601
(.107))
Tibouchina . . .. .. . 0.833
(.310)
0.821
(.230)
0.875
(.392)
ns
Woody:Clidemia . . .. .. . .. . 0.914
(.183)0.405(.065)
0.502(.064)
*
Miconia . . . .. . .. . . . 0.927(.067)
0.441(.020)
0.946(.030)
*
Note. P indicates the significance of the one-way ANOVA on thedifference between the light treatments. Symbols as in table 1. Datafor high-light Arthrostema are from greenhouse measurements.
other melastomes (Denslow et al. 1990) and tropical trees(Thompson et al. 1992). This reduction of leaf Nmass was prob-ably caused by a large decrease in SLM that accompaniedgrowth under LL, as discussed in Plant Size, Leaf Numberand Area, and Specific Leaf Mass. The herbs were more ef-ficient in absorbing and using N than the woody species, con-
sistent with their higher RGR (Lambers and Poorter 1992).Under similar conditions of full sunlight and watering, themelastomes exhibited higher leaf N and P concentration thana large group of Hawaiian native species (melastomes,
; natives, N, and melastomes2.11 0.30 1.36% 0.63%
; natives, P) (Baruch and Gold-0.31 0.11 0.08% 0.05%
stein 1999). Considered in isolation, this would make invasivespecies less efficient in nutrient use. However, a large foliar Ncontent promotes carbon assimilation and enhances thegrowth potential of invasive species (Field and Mooney 1986;Baruch and Goldstein 1999).
Leaf reflectance did not show any particular trend in re-sponse to light levels in any species. The significant increase
in leaf transmittance in all species under LL was most likelya consequence of the parallel decrease in SLM already dis-cussed. This increase resulted in a decrease in leaf absorbanceunder LL except in Arthrostema. This was unexpected, as in-creased absorbance should be a valuable response to low lightavailability. The two herbs from open habitats had lower leafabsorbances than the woody shade-tolerant plants. These re-sults are different than results obtained by Lee and Graham(1986), who found no significant difference in leaf absorbancebetween a large group of tropical sun and shade plants. Thedeep green leaves of Miconia with purple underside were themost absorptive of all, as would be expected from this shade-tolerant species (Lee and Graham 1986). The higher leaf re-flectance and lower leaf absorbance of the herbs probably helpin reducing the radiation load and transpirational water lossof these open habitat species (Nobel 1991).
Leaf CC was similar to other herbs and woody plants(Poorter and Villar 1997). Both woody species had higher leafCC than Tibouchina, as expected from their denser and pos-sibly longer-lived leaves. Although the differences in CC weresmall, they can lead to moderate differences in RGR (Poorterand Villar 1997). The relatively high leaf CC in Arthrostemacould be attributed to the presumably high energy content ofthe sticky secretions produced by its leaf glandular hairs thatmay act as herbivory deterrents. Leaf CC decreased from HLto LL in all species. This could be the result of the thinner andless dense leaves produced under LL with the concurrent prob-
able decrease of high-energy lignin content (Poorter 1994).Higher CC of sun leaves was also found for a group of tropicalPiper species and other plants (Williams et al. 1989; Poorterand Villar 1997). Under similar light conditions, leaf CC ofthe melastomes was lower than that for a large group of nativeHawaiian species (melastomes, ; natives,1.25 0.06 1.39
equivalents of glucose g1) (Baruch and Goldstein 1999).0.01Low leaf CC has been associated with plants with high RGR(Lambers and Poorter 1992; Poorter and Villar 1997). Thelower leaf CC of the melastomes supports that these speciesuse carbon more efficiently than native species by investingless energy per unit of leaf produced.
-
7/28/2019 Baruch Et Al 2000
12/13
BARUCH ET AL.LIFE HISTORY OF INVASIVE MELASTOMES 117
Gas Exchange
The maximum A values obtained were in the range of thosereported for a large group of early and late successional trop-ical plants grown under contrasting light regimes (Strauss-Debenedetti and Bazzaz 1996). However, A of Miconia cal-vescens was higher than for other Miconias in the field (Den-slow et al. 1990; Newell et al. 1993; Ellsworth and Reich1996). On an area and mass basis, A was highest in the herbs.On an area basis, A decreased monotonically as light intensitydecreased, whereas it was maintained or even increased whenconsidered on a mass basis. This is likely to be a consequenceof the decrease in SLM from HL to LL. No substantial dif-ferences were observed in the shape of the light curves amongthe shade-tolerant Miconia, the shade-intolerant Tibouchina,and the intermediate Clidemia. Theterm acclimation as usedhere is similar to that of plasticity in Stauss-Debenedetti andBazzaz (1996), in that it refers to the range of phenotypicexpression under constant contrasting environments. All spe-
cies showed light acclimation, i.e., light responses were pro-portional to the light regime in which they were grown (Bjork-man 1981). This was expected for Miconia and Clidemia, asthey grow under shade and in openings in the field. This plas-ticity is even more valuable for Miconia, as it switches froma seedling and sapling in the forest understory to the full-lightconditions in the canopy. This acclimation potential ofMiconiawas similar to that observed in other melastomes, tropicalplants, and invaders in Hawaii (Denslow et al. 1990; Newellet al. 1993; Ellsworth and Reich 1996; Pattison et al. 1998).Under high-light conditions, the A of the melastomes washigher than that of a large group of Hawaiian native herbs,shrubs and trees (melastomes, mmol m2 s1; na-8.47 1.49tives, mmol m2 s1) (Baruch and Goldstein 1999).6.9 4.62
The higher A of the melastomes was strongly related to theirhigh RGR. Both herbs had a significantly higher PNUE thanthe woody species, which is the likely result of the higher Aand lower SLM of the former. Under similar high-light con-ditions, PNUE of the melastomes was up to 400% higher thanfor a large group of native Hawaiian species (Baruch and Gold-
stein 1999).
Conclusion
In all species, all variables responded strongly to light lim-itation, whereas the responses to water stress were weaker butinteracted with light responses. Quantitatively, biomass allo-cation, RGR, SLM, and CC of herbs responded more to thedifferent treatments than the woody plants. All species showedacclimation to light. With few exceptions, the responses of thefour melastomes to light stress were similar to those of othertropical plants. When compared with a large number of Ha-waiian native species (Pattison et al. 1998; Baruch and Gold-stein 1999), the four melastomes had higher A and RGR and
lower SLM and CC. These traits probably contribute signifi-cantly to the success of the invader melastomes in the disturbedmesic habitats of Hawaii. We hope that the results of this studywill provide insights that might help land managers seekingto control these and other similar invasive species
Acknowledgments
Z. Baruch thanks Universidad Simon Bolvar for supportduring sabbatical leave at the University of Hawaii. Researchfunds were provided by the University of Hawaii CooperativeNational Park Resources Studies Unit. We thank Dr. CliffordSmith for support during the study.
Literature Cited
Almeda F 1990 Melastomataceae. Pages 903917 in DL Wagner, DR
Herbst, SH Sohmer, eds. Manual of the flowering plants of Hawaii.
University of Hawaii Press and Bishop Museum Press, Honolulu.
Baruch Z, G Goldstein 1999 Leaf construction cost, nutrient concen-
tration, and net CO2 assimilation of native and invasive species in
Hawaii. Oecologia 121:183192.
Baruch Z, MM Ludlow, R Davis 1985 Photosynthetic responses of
native and introduced C4 grasses from Venezuelan savannas. Oeco-
logia 67:288293.
Bazzaz FA 1986 Life history of colonizing plants: some demographic,
genetic and physiological features. Pages 96110 in HA Mooney,
JA Drake, eds. Ecology of biological invasions of North America
and Hawaii. Ecological Studies 58. Springer, New York.Bjorkman O 1981 Responses to different quantum flux densities.
Pages 57107 in OL Lange, PS Nobel, CB Osmond, H Ziegler, eds.
Physiological plant ecology. Vol 1. Responses to the physical envi-
ronment. Springer, Berlin.
Chapin FS 1991 Effects of multiple environmental stresses on nutrient
availability and use. Pages 6788 in HA Mooney, WE Winner, EJ
Pell, eds. Response of plants to multiple stresses. Academic Press,
San Diego, Calif.
Chazdon RL, CB Field 1987 Determinants of photosyntheticcapacity
in six rainforest Piper species. Oecologia 73:222230.
Chazdon R, R Pearcy, D Lee, N Fetcher 1996 Photosynthetic re-
sponses of tropical forest plants to contrasting light environments.
Pages 555 in SS Mulkey, RL Chazdon, AP Smith, eds. Tropicalforest plant ecophysiology. Chapman & Hall, New York.
Denslow JS, JC Schulz, PM Vitousek, BR Strain 1990 Growth re-
sponses of tropical shrubs to treefall gap environments. Ecology 71:
165179.
Ellison AM, JS Denslow, BA Loiselle, D Brenes 1993 Seed and seed-
ling ecology of neotropical Melastomataceae. Ecology 74:
17331749.
Ellsworth DS, PB Reich 1996 Photosynthesis and leaf nitrogen in five
Amazonian tree species during early secondary succession. Ecology
77:581594.
Fetcher N, SF Oberbauer, G Rojas, BR Strain 1987 Efectos del re-
gimen de luz sobre la fotosntesis y crecimiento en plantulas dearboles de un bosque lluvioso tropical de Costa Rica. Rev Biol Trop
35(suppl):97110.
Field CB, HA Mooney 1986 The photosynthesis-nitrogen relation-
ships in wild plants. Pages 2555 in TJ Givnish, ed. On the economy
of plant form and function. Cambridge UniversityPress, Cambridge.
Isaac RA, WC Johnson 1983 High speed analysis of agriculture sam-
ples using inductivelycoupled plasma-atomic emission spectroscopy.
Spectrochim Acta 38B:277282.
Kitajima K 1996 Ecophysiology of tropical tree seedlings. Pages
559596 in SS Mulkey, RL Chazdon, AP Smith, eds. Tropical forest
plant ecophysiology. Chapman & Hall, New York.
Lambers H, H Poorter 1992 Inherent variation in growth rate be-
-
7/28/2019 Baruch Et Al 2000
13/13
118 INTERNATIONAL JOURNAL OF PLANT SCIENCES
tween higher plants: a search for physiological causes and ecologicalconsequences. Adv Ecol Res 23:188261.
Lee DW, K Baskaran, M Mansor, H Mohamad, SK Yap 1996Irradiance and spectral quality affect Asian tropical rain forest treeseedling development. Ecology 77:568580.
Lee DW, R Graham 1986 Leaf optical properties of rainforest sunand extreme shade plants. Am J Bot 73:11001108.
Loope LL, D Mueller-Dombois 1989 Characteristics of invaded is-lands, with special reference to Hawaii. Pages 257280 inJA Drake,HA Mooney, F Di Castri, RH Groves, FJ Kruger, M Rejmanek, MWilliamson, eds. Biological invasions: a global perspective. ScientificCommittee on Problems of the Environment (SCOPE). Vol 37. Wi-ley, Chichester.
Medeiros AC, LL Loope, P Conant, S McElvaney 1997 Status, ecol-ogy and management of the invasive plant Miconia calvescens DC(Melastomataceae) in the Hawaiian Islands. Bishop Mus Occas Pap48:2336.
Meyer JY, J Florence 1996 Tahitis native flora endangered by theinvasion of Miconia calvescens. J Biogeogr 23:775781.
Meyer JY, JP Malet 1997 Study and management of the alien invasivetree Miconia calvescens DC (Melastomataceae) in the islands ofRaiatea and Tahaa (Society Islands, French Polynesia), 19921996.
Technical Report 111. Hawaii Cooperative National ParkResourcesStudies Unit, Honolulu. 56 pp.
Mulkey SS, RL Chazdon, AP Smith, eds 1996 Tropical forest plantecophysiology. Chapman & Hall, New York.
Nelson DW, LE Sommers 1972 Determination of total nitrogen inplant material. Agron J 65:109112.
Newell EA, EP McDonald, BR Strain, JS Denslow 1993 Photo-synthetic responses ofMiconia species to canopy openings in a trop-ical lowland rainforest. Oecologia 94:4956.
Nobel PS 1991 Physicochemical and environmental plant physiology.Academic Press, San Diego, Calif. 635 pp.
Orians GH, OT Solbrig 1977 A cost-income model of leaves androots with special reference to arid and semi-arid areas. Am Nat111:677690.
Pattison RR, G Goldstein, A Ares 1998 Growth, biomass allocation
and photosynthesis of invasive and native Hawaiian rainforest spe-cies. Oecologia 117:449459.
Pearcy RW, DA Sims 1994 Photosynthetic acclimation to changinglight environments: scaling from the leaf to the whole plant. Pages145174 in MM Caldwell, RW Pearcy, eds. Exploitation of envi-ronmental heterogeneity by plants. Academic Press, San Diego,Calif.
Poorter H 1994 Construction costs and payback time of biomass: awhole plant perspective. Pages 111127 in J Roy, E Garnier, eds. Awhole plant perspective on carbon-nitrogen interactions. SPB Aca-demic, The Hague.
Poorter H, R Villar 1997 The fate of acquired carbonin plants: chem-ical composition and construction costs. Pages 3972 in FA Bazzaz,
J Grace, eds. Plant resource allocation. Academic Press, San Diego,Calif.
Rejmanek M, Richardson D 1996 What attributes make some plantspecies more invasive? Ecology 77:16551661.
Schulze ED 1981 Plant lifeformsand their carbon, water andnutrientrelations Pages 615676 in LO Lange, PC Nobel, CB Osmond, HZiegler, eds. Encyclopedia of plant physiology. Vol 12B. Springer,Berlin.
Schulze ED, FS Chapin 1987 Plant specialization to environments ofdifferent resource availability. Pages 120148 in DE Schulze, HZwolfer, eds. Potentials and limitations of ecosystem analysis. Eco-logical Studies 61. Springer, Berlin.
Smith CW 1992 Distribution, status, phenology, rate of spread, andmanagement of Clidemia in Hawaii. Pages 241253 in CP Stone,
CW Smith, JT Tunison, eds. Alien plant invasions in native ecosys-tems of Hawaii. University of Hawaii Press, Honolulu.
Strauss-Debenedetti S, FA Bazzaz 1996 Photosyntheticcharacteristics
of tropical trees along successional gradients. Pages 162186 in SS
Mulkey, RL Chazdon, AP Smith, eds. Tropical forest plant ecophys-
iology. Chapman & Hall, New York.
SYSTAT 1997 SYSTAT version 7.0 for Windows. SPSS, Chicago.
Thompson WA, PE Kriedemann, IE Craig 1992 Photosynthetic re-
sponse to light and nutrients in sun-tolerant and shade-tolerantrain-
forest trees. I. Growth, leaf anatomy and nutrient content. Aust JPlant Physiol 19:118.
Vitousek PM 1994 Beyond global warming: ecology and global
change. Ecology 75:18611876.
Vitousek PM, LL Loope, CP Stone 1987 Introduced species in Ha-
waii: biological effects and opportunities for ecological research.
Trends Ecol Evol 2:224227.
Waring RH 1991 Responses of evergreen trees to multiple stresses.
Pages 371390 in HA Mooney, WE Winner, EL Pell, eds. Response
of plants to multiple stresses. Academic Press, San Diego, Calif.
Wester L 1992 Origin and distribution of adventive alien flowering
plantsin Hawaii. Pages 99154 in CP Stone, CW Smith,JT Tunison,
eds. Alien plant invasions in native ecosystems of Hawaii. University
of Hawaii Press, Honolulu.
Williams DG, RA Black 1994 Drought response of a native and in-
troduced Hawaiian grass. Oecologia 97:512519.
Williams KF, CB Field, HA Mooney 1989 Relationships among leaf
construction cost, leaf longevity, and light environmentin rain-forest
plants of the genus Piper. Am Nat 133:198211.
Williams KF, F Percival, J Merino, HA Mooney 1987 Estimation oftissue construction cost from heat of combustion and organic ni-
trogen content. Plant Cell Environ 10:725734.